Abstract
α-Amylase production by thermophilic Bacillus sp strain SMIA-2 cultivated in liquid cultures containing soluble starch as a carbon source and supplemented with 0.05% whey protein and 0.2% peptone reached a maximum activity at 32 h, with levels of 37 U/mL. Studies on the amylase characterization revealed that the optimum temperature of this enzyme was 90°C. The enzyme was stable for 1 h at temperatures ranging from 40-50°C while at 90°C, 66% of its maximum activity was lost. However, in the presence of 5 mM CaCl2, the enzyme was stable at 90°C for 30 min and retained about 58% residual activity after 1 h. The optimum pH of the enzyme was found to be 8.5. After incubation of enzyme for 2 h at pH 9.5 and 11.0 was observed a decrease of about 6.3% and 16.5% of its original activity. At pH 6.0 the enzyme lost about 36% of its original activity. The enzyme was strongly inhibited by Co2+, Cu2+ and Ba2+, but less affected by Mg2+, Na+ and K+. In the presence of 2.0 M NaCl, 63% of amylase activity was retained after 2 h incubation at 45°C. The amylase exhibited more than 70% activity when incubated for 1 h at 50°C with sodium dodecyl sulphate. However, very little residual activity was obtained with sodium hypochlorite and with hydrogen peroxide the enzyme was completely inhibited. The compatibility of Bacillus sp SMIA-2 amylase with certain commercial detergents was shown to be good as the enzyme retained 86%, 85% and 75% of its activity after 20 min incubation at 50°C in the presence of the detergent brands Omo®, Campeiro® and Tide®, respectively.
Keywords: α-Amylase, Thermophilic bacterium, Bacillus sp
INTRODUCTION
The majority of industrial enzymes used currently belong to the hydrolase group, which are active on many natural substrates. For years microorganisms have been the principal source of many different enzymes, which were identified after extensive research and currently find their main uses in industrial applications (5).
α-Amylase (EC 3.2.1.1) is an important enzyme used in the industry and accounts for approximately 25% of the enzyme market (24). Thermostable α-amylases have extensive commercial applications in starch processing, brewing and sugar production, desizing in textile industries and in detergent manufacturing processes (10,15).
Amylases can be obtained from several sources (1, 26). They are usually produced by bacteria belonging to the genus Bacillus for industrial applications such as B. amyloliquefaciens, B. stearothermophilus, B. subtilis and Bacillus licheniformis (22), the latter now being of greater industrial importance (7). The properties of each α-amylase such as thermostability, pH profile, pH stability, and Ca-independency must be matched to its application. For example, α-amylases used in starch industry must be active and stable at low pH but in detergent industry at high pH values (19).
In this article, the properties of an thermostable α-amylase produced by the thermophilic Bacillus sp strain SMIA-2, isolated from soil in Campos dos Goytacazes City, Rio de Janeiro, Brazil, are reported.
MATERIALS AND METHODS
Microorganism and culture conditions
The bacterial strain used in this study was Bacillus sp SMIA-2, a thermophilic strain isolated from a local soil sample (20). Production of α-amylase was carried out in a medium containing (g/L of distilled water): KCl-0.3, MgSO4-0.5, K2HPO4-0.87, CaCl2-2.2x10-3, ZnO-2.5x10-3, FeCl3.6H2O-2.7x10-2, MnCl2.4H2O-1.0x10-2, CuCl2.2H2O-8.5x10-4, CoCl2.6H2O-2.4x10-3, NiCl3.6H2O-2.5x10-4, H3BO3-3.0x10-4, whey protein-0.5, peptone-2.0 and soluble starch-5.0. The pH was adjusted to 7.5 with NaOH and this basal medium was sterilized by autoclaving at 121°C for 15 minutes. The medium (25 mL in 250 mL Erlenmeyer flasks) was inoculated with 1 mL of an overnight culture and incubated at 50°C in a orbital shaker (Thermo Forma, Ohio, USA) operated at 180 min-1 for 48 h. Triplicate aliquots withdraw at regular intervals and analysed for growth (OD 600 nm) and pH. The cells and residues were removed from the culture broth by centrifugation and concentrated by precipitating the enzyme with ammonium sulfate (60% saturation). The precipitate was dissolved and dialyzed overnight against 10 mM phosphate buffer, pH 7.0. The dialyzed enzyme was then concentrated by lyophilization and used for subsequent studies.
Amylase assay
The activity of α-amylase was assayed by incubating 0.5 mL of the diluted enzyme (0.55 mg.mL-1) with 0.5 mL soluble starch (0.5%, w/v) prepared in 0.05 M Tris-HCl buffer, pH 8.5. After incubation at 90°C for 10 minutes the reaction was stopped and the reducing sugars released were assayed colorimetrically by the addition of 1 mL of 3-5-dinitrosalicylic acid reagent (16). One enzyme activity unit (U) was defined as the amount of enzyme releasing 1 μmol of glucose from the substrate in 1 minute at 90°C.
Effect of the pH
The effect of pH on the activity of α-amylase was measured by incubating 0.5 mL of the diluted enzyme (0.55 mg.mL-1) and 0.5 mL of buffers presenting pH from 6.0 to 12.0, containing 0.5% soluble starch for 10 minutes at 90°C. The buffers used were: phosphate buffer, pH 6.0-8.0; Tris-HCl buffer, pH 8.5-9.0 and glycine-NaOH, pH 10.0-12.0. The stability of the enzyme at different pH values was also studied by incubating the enzyme at various pH values ranging from 6.0 to12.0 for 2 h and then estimating the residual activity.
Effect of temperature
The effect of temperature on the enzyme activity was determined by performing the previously described standard assay procedure for 10 minutes at pH 8.5 within a temperature range of 40-100°C. Thermostability was determined by incubation of the lyophilized enzyme at temperatures ranging from 40-100°C for 1 h in a constant-temperature water bath. After treatment the residual enzyme activity was assayed.
Effect of metal ions
The effect of metal ions on α-amylase activity was measured incubating the enzyme at 90°C for 2 min with various metal ions at a concentration of 5 mM and 10 mM. The enzyme assay was carried out in the presence of Ca2+, Zn2+, Mn2+, Ni2+ and Ba2+ chlorides, and Mg2+, Fe2+, Co2+ and Cu2+ sulphates.
Salt tolerance test
The enzyme was incubated in 50 mM Tris-HCl buffer (pH 8.5) containing NaCl at concentrations of 0 to 5 M for 120 minutes at 45°C. The activity of the enzyme was measured in the same way as mentioned earlier.
Effect of inhibitors and oxidizing agents
The enzyme was incubated with 5 mM sodium dodecylsulphate (SDS), sodium hypochlorite (NaClO), hydrogen peroxide (H2O2) and EDTA (Ethylene diaminetetraacetic acid) at 50°C for 1 h. After incubation, the residual activity was determined by the standard enzyme assay. The results were recorded as the percentage of residual activity calculated with reference to activity controls incubated in the absence of these compounds.
Evaluation of enzyme for use in detergent formulation
The detergent brands used were Ariel®, Biz®, Cheer®, Tide®, Campeiro® and Omo®. They were diluted in double distilled water to a final concentration of 7 mg.mL-1 to simulate washing conditions and heated at 100°C for 15 minutes to inactivate the enzymes that could be part of their formulation. The detergents were added to the reaction mixture and the reaction was carried out under standard assay conditions. To determine the stability of Bacillus sp SMIA-2 amylase in the presence of the different detergents, an amylase concentration of 1 mg.mL-1 was added in detergent solution and incubated at 50°C for 60 minutes. Aliquots (0.5 mL) were taken at different time intervals and the residual activity determined at 90°C and compared with the control sample incubated at 50°C without any detergent (2,21).
RESULTS AND DISCUSSION
Enzymatic production
Fig. 1 shows the time-course profile of amylase production by Bacillus sp strain SMIA-2 in basal medium containing soluble starch (0.5%) as a carbon source and supplemented with whey protein (0.05%) and peptone (0.2%). α-amylase production by Bacillus sp strain SMIA-2 began in the exponential growth phase, reaching a maximum at 32 h, with levels of 37 U/mL. Subsequently amylase levels remained more or less the same up to 36 h and then dropped to 30 U/mL at 48 h. The pH of the medium dropped as cells started to grow, but as soon as enzyme production was initiated, the pH started to rise. This may indicate that some organic nitrogen was being consumed. The end of enzyme production was signalled by a slight decrease of pH. Thus the pH profile provides a useful means of monitoring the production process.
Figure 1.
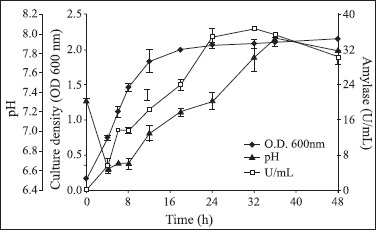
Time course of α-amylase production by Bacillus sp strain SMIA-2 grown at 50°C on soluble starch (0.5%) in shake flasks. Results represent the means of three separate experiments, and bars indicated ± 1 standard deviation. Absence of bars indicates that errors were smaller than symbols.
Effect of the pH
The effect of pH on α-amylase activity as a function of pH is shown in Fig. 2. Optimum pH was found to be 8.5. The enzyme activity at pH 7.0 and 11.0 were 72% and 81.4% of that at pH 8.5, respectively. After incubation of the enzyme solution for 2h at pH 6.0-12.0, the original activity at pH 9.5 decreased by 63% and at pH 11.0 the decrease was 16.5%. However, at pH 6.0, the original activity decreased 36%. These results suggest that the activity of the enzyme is higher in alkaline pH, making this enzyme attractive for the detergent industry. α-amylases produced by other Bacillus sp have shown optimum activities at pH values as low as 3.5 or as high as 12 (3,13).
Figure 2.
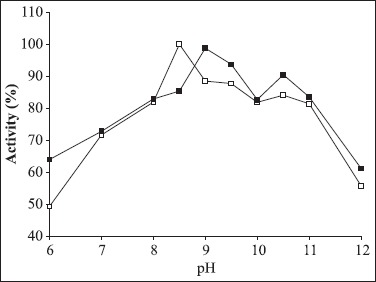
Optimum pH (□) and stability (■) of α-amylase produced by Bacillus sp strain SMIA-2 grown at 50°C for 48 h. Relative activity is expressed as a percentage of the maximum (100% of enzyme activity = 36.1U/mL).
Effect of temperature
Fig. 3 shows the activity of the lyophilized enzyme preparation assayed at temperatures ranging from 40°C-100°C at pH 8.5 and a substrate concentration of 0.5%. Enzyme activity increased with temperature within the range of 40°C to 90°C. A reduction in enzyme activity was observed at values above 90°C. The optimum temperature of this α-amylase was 90°C, which was higher or similar to that described for other Bacillus α-amylases (4,6,12,13,24).
Figure 3.
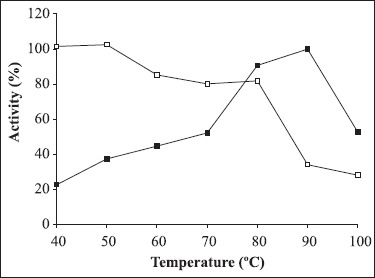
Optimum temperature (■) and stability temperature (□) of α-amylase produced by Bacillus sp strain SMIA-2 grown at 50°C for 48 h. Relative activity is expressed as a percentage of the maximum. 100% of enzyme activity = 36.4 U/mL.
The residual activities of crude amylase incubated at different temperatures for a period of 1 h were estimated at optimum temperature. The enzyme was stable for 1 h at temperatures ranging from 40-50°C while at 80°C, 18% of its maximum activity was lost. At 90°C, 66% of its maximum activity was lost, however, the addition of 5 mM CaCl2 improved the thermostability of the amylase, as indicated in Fig. 4. The enzyme was stable at 90°C for 30 min and retained about 50.6% residual activity after 1 h. The requirement of Ca2+ by α-amylases for their stability at higher temperature has also been reported for Bacillus sp. I-3 (9), Bacillus sp. ANT-6 (6), B. subtilis (18), Bacillus clausii BT-21 (8), and Bacillus licheniformis (14). The stabilizing effect of Ca2+ on thermostability of the enzyme can be explained by the salting out of the hydrophobic residues by Ca2+ in the protein, thus causing the adoption of a compact structure (27).
Figure 4.
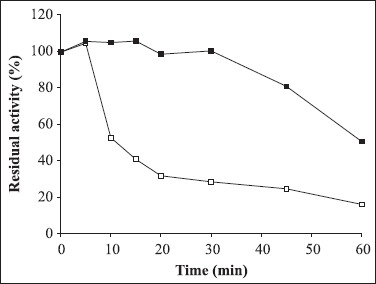
Termostability of amylase at 90°C in the presence (■) or absence of Ca2+ (□). 100% of enzyme activity = 50.5 U/mL.
Effect of metal ions
The amylase did not require any specific ion for catalytic activity (Fig. 5). Najafi et al. (2005) observed that the α-amylase from Bacillus subtilis AX20 did not have an obligate requirement for divalent metal ions to be active and its activity was not stimulated in the presence of metal ions. A stronger inhibitory effect was observed in the presence of Cu2+, Co2+, Ba2+ and Mn2+. On the other hand, no significant inhibition of activity was observed in the presence of 5 mM of Mg2+, Na+, Zn2+ and K+. Some amylases are metalloenzymes, containing a metal ion with a role in catalytic activity. The inhibition of Bacillus sp strain SMIA-2 amylase by Co2+, Cu2+ and Ba2+ ions could be due to competition between the exogenous cations and the protein-associated cation, resulting in decreased metalloenzyme activity (15).
Figure 5.
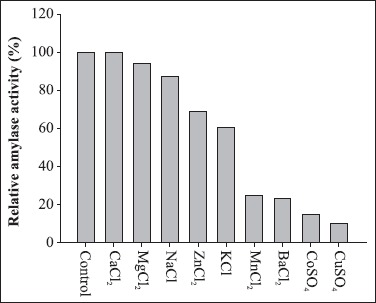
Effect of metal ions on amylase activity. The activity is expressed as a percentage of the activity level in the absence of metal ion. 100% of enzyme activity = 40.62 U/mL.
Salt tolerance test
In the presence of 2.0 M NaCl, 63.4% of amylase activity was retained after 2 h incubation at 45°C. The α-amylase produced by Bacillus sp. MD 124 was stable in NaCl solution and retained 75% of its original activity in 5 M after 24 h of incubation (11).
Effect of inhibitors and some oxidizing agents on enzyme activity and compatibility with various commercial detergents
Besides pH and temperature stability, a good detergent amylase should also be stable to various detergent ingredients, such as surfactants, chelators and oxidants (23). The amylase from Bacillus sp SMIA-2 retained more than 70% activity after incubated for 1 h at 50°C with sodium dodecyl sulphate, an anionic detergent. However, very little residual activity was obtained with sodium hypochlorite. The enzyme was completely inhibited by hydrogen peroxide. The amylase from Bacillus sp. PN5 retained more than 80% activity when incubated with sodium perborate and sodium dodecyl sulphate and more than 70% activity when incubated with hydrogen peroxide for an hour. However, very little residual activity was obtained with sodium hypochlorite (23). Regarding to the effect of EDTA, the enzyme retained 53% of its activity when incubated for 20 min at 50°C. The α-amylase from Bacillus sp PS-7 retained almost 100% activity when 1mM EDTA was added to the reaction mixture (25).
Figure 6.
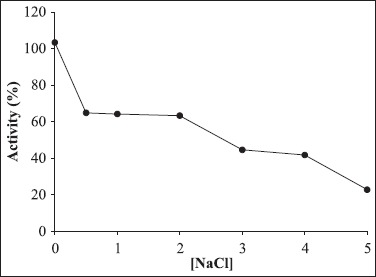
Effect of NaCl concentration on α-amylase produced by Bacillus sp strain SMIA-2 grown at 50°C for 24 h. Relative activity is expressed as a percentage of the maximum. 100% of enzyme activity = 34.8 U/mL.
Figure 7.
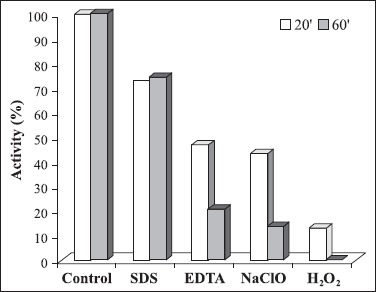
Effect of inhibitors and oxidizing agents on α-amylase activity. The activity is expressed as a percentage of the activity level in the absence of inhibitors and oxidizing agents. 100% of enzyme activity = 31.9 U/mL.
Studies on the effect of detergents on amylase activity (Fig. 8a) show that the enzyme activity increased in the presence of Omo® and was almost the same as that of the control in the presence of Campeiro®. On the other hand, the enzyme was severely inhibited in the presence of Biz® and Cheer®. The compatibility of Bacillus sp. SMIA-2 amylase with certain commercial detergents was shown to be good as the enzyme retained 86%, 85% and 75% of its activity after 20 minutes incubation at 50°C in the presence of the detergent brands Omo®, Campeiro® and Tide® respectively (Fig. 8b). After 40 minutes, the enzyme retained 49%, 47% and 40% activity at 50°C in the presence of the detergents Tide®, Omo® and Campeiro® respectively.
Figure 8.
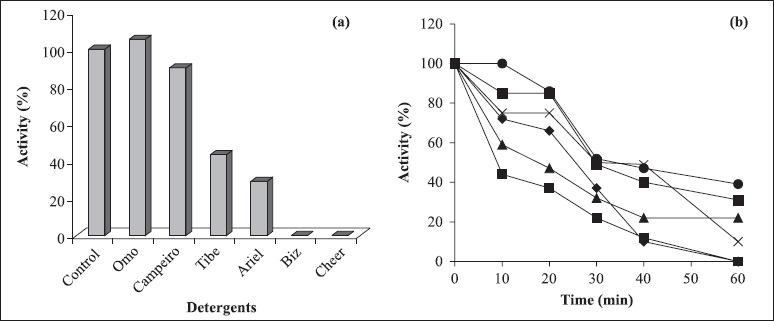
Compatibility of α-amylase activity from Bacillus sp. SMIA-2 with commercial detergents (♦ Ariel®, ■ Biz®, Δ Cheer®, x Tide®, ▅ Campeiro®, ● Omo®). The activity is expressed as a percentage of the activity level in the absence of detergents (100% of enzyme activity = 47 U/mL).
ACKNOWLEDGMENTS
The authors are highly thankful to the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro for financial support.
RESUMO
Propriedades de uma amilase de um termofílico Bacillus sp
A produção de α-amilase por um termofilico, Bacillus sp SMIA-2, cultivado em meio líquido contendo amido solúvel como fonte de carbono, alcançou uma atividade máxima de 37 U/mL em 32 horas. Estudos sobre a caracterização da amilase revelaram que a temperatura ótima desta enzima foi 90°C. A enzima foi estável por 1 hora a temperaturas de 40 e 50°C enquanto a 90°C, 66% da atividade máxima foi perdida. Entretanto, na presença de 5 mM de CaCl-2, a enzima foi estável a 90°C por 30 minutos e manteve cerca de 58% de sua atividade residual por 1 hora. O pH ótimo da enzima encontrado foi de 8.5. Após a incubação da enzima por 2 horas a pH 9.5 e 11.0 foi observado um decréscimo de aproximadamente 6.3% e 16.5% da atividade original. Em pH 6.0 a enzima perdeu cerca de 36% de sua atividade original. A enzima foi fortemente inibida por Co2+, Cu2+, e Ba2+, porém pouco afetada por Mg2+, Na+ e K+. Na presença de 2.0 M de NaCl, 63% da atividade da amilase foi mantida após 2 horas de incubação a temperatura de 45°C. A amilase exibiu atividade acima de 70% quando incubada por 1 hora a 50°C em presença de sódio dodecil sufato (SDS). Entretanto, uma baixa atividade residual foi obtida quando na presença do hipoclorito de sódio e uma completa inibição quando a enzima foi incubada em peróxido de hidrogênio. A compatibilidade da amilase produzida pelo Bacillus sp SMIA-2, em relação a alguns detergentes comerciais mostrou que a enzima manteve 86%, 85%, e 75% da atividade após 20 minutos de incubação a 50°C na presença dos detergentes Omo®, Campeiro® e Tide®, respectivamente.
Palavras-chave: α-Amilase, Bactéria termofílica, Bacillus sp.
REFERENCES
- 1.Aquino A.C.M.M., Jorge J.A., Terenzi H.F., Polizeli M.L.T.M. Studies on a thermostable α-amylase from the thermophilic fungus Scytalidium thermophilum. Appl. Microbiol. Biotechnol. 2003;61:323–328. doi: 10.1007/s00253-003-1290-y. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee U.C., Sani R.K., Azmi W., Soni R. Thermoestable alkaline protease from Bacillus brevis and its characterization as a laundry detergent additive. Process Biochem. 1999;35:213–219. [Google Scholar]
- 3.Bernhardsdotter E.C.M.J., Ng J.D., Garriott O.K., Pusey M.L. Enzymic properties of an alkaline chelator-resistant α-amylase from an alkaliphilic Bacillus sp. isolate L1711. Process Biochem. 2005;40:2401–2408. [Google Scholar]
- 4.Bertoldo C., Antranikian G. Starch hydrolyzing enzymes from thermophilic archae and bacteria. Curr. Opin. Chem. Biol. 2002;6:151–160. doi: 10.1016/s1367-5931(02)00311-3. [DOI] [PubMed] [Google Scholar]
- 5.Bon E.P.S. A tecnologia enzimática no Brasil. ENZITEC. 1995;95:9–14. [Google Scholar]
- 6.Burhan A., Nisa U., Gokhan C., Omer C., Ashabil A., Osman G. Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp. isolate ANT-6. Process Biochem. 2003;38:1397–403. [Google Scholar]
- 7.Declerck N., Machius M., Wiegand G., Huber R., Gaillardin C. Probing structural determinants specifying high thermostability in Bacillus licheniformis α-amylase. J. Mol. Biol. 2000;31:1041–1057. doi: 10.1006/jmbi.2000.4025. [DOI] [PubMed] [Google Scholar]
- 8.Duedahl-Olesen L., Kragh K.M., Zimmermann W. Purification and characterisation of a malto-oligosaccharide-forming Amylase from amylase active at high pH from Bacillus clausii BT-21. Carbohydr. Res. 2000;329:97–107. doi: 10.1016/s0008-6215(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 9.Goyal N., Gupta J.K., Soni S.K. A novel raw starch digesting thermostable α-amylase from Bacillus sp.I-3 and its use in the direct hydrolysis of raw potato starch. Enzyme and Microb. Technol. 2005;37:723–734. [Google Scholar]
- 10.Hendriksen H.V., Pedersen S., Bisgard-Frantzen H. A process for textile warp sizing using enzymatically modified starches. Patent Application. WO 99/35325. [Google Scholar]
- 11.Jana M., Pati B. Thermostable, salt-tolerant α-amylase from Bacillus sp. MD-124. J. Bras. Microbiol. 1997;37:323–326. [Google Scholar]
- 12.Jin F., Li Y., Zhang C., Yu H. Thermostable α-amylase and a-galactosidase production from the thermophilic and aerobic Bacillus sp. JF strain. Process Biochem. 2001;36:559–564. [Google Scholar]
- 13.Konsula Z., Liakopoulou-Kyriakides M. Hydrolysis of starches by the action of an α-amylase from Bacillus subtilis. Process Biochem. 2004;39:1745–1749. [Google Scholar]
- 14.Krishnan T., Chandra A.K. Purification and characterization of α-amylase from B. licheniformis CUMC 305. Appl. Environ. Microbiol. 1983;46:430–437. doi: 10.1128/aem.46.2.430-437.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lévêque E., Janecek S., Haye B., Belarbi A. Thermophilic archaeal amylolytic enzymes. Enzyme Microbiol. Technol. 2000;26:3–14. [Google Scholar]
- 16.Miller G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal. Chem. 1959;3:426–428. [Google Scholar]
- 17.Najafi M.F., Deobagkar D., Deobagkar D. Purification and characterization of an extracellular α-amylase from Bacillus subtilis AX20. Protein Expr. and Purif. 2005;41(2):349–354. doi: 10.1016/j.pep.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen A.D., Pusey M.L., Fuglsang C.C., Westh P. A proposed mechanism for the thermal denaturation of a recombinant Bacillus halmapalus α-amylase the effect of calcium ions. Biochim. Biophys. Acta. 2003;1652:52–63. doi: 10.1016/j.bbapap.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen J.E., Borchert T.V. Protein engineering of bacterial α-amylases. Biochim Biophys. Acta. 2000;1543:253–274. doi: 10.1016/s0167-4838(00)00240-5. [DOI] [PubMed] [Google Scholar]
- 20.Nunes A.S., Martins M.L.L. Isolation, properties and kinetics of growth of a thermophilic Bacillus. Braz. J. Microbiol. 2001;32:271–275. [Google Scholar]
- 21.Phadatare S.U., Deshpande V.V., Srinivasan M.C. High activity alkaline protease from Conidiobolus coronatus (NCL 86.8.20): Enzyme production and compatibility with commercial detergents. Enzyme Microbiol. Technol. 1993;15:72–76. [Google Scholar]
- 22.Sajedi R.H., Naderi-Manesh H., Khajeh K., Ahmadvand R., Ranjbar B., Asoodeh A., Moradian F. A Ca-independent α-amylase that is active and stable at low pH from the Bacillus sp. KR-8104. Enzyme and Microb. Technol. 2005;36:666–671. [Google Scholar]
- 23.Saxena R.K., Dutt K., Agarwal L., Nayyar P. A highly thermostable and alkaline amylase from a Bacillus sp. PN5. Bioresour. Technol. 2007;98(2):260–265. doi: 10.1016/j.biortech.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Sidhu G.S., Sharma P., Chakrabarti T., Gupta J.K. Strain improvement for the production of a thermostable α-amylase. Enzyme and Microb. Technol. 1997;21:525–530. [Google Scholar]
- 25.Sodhi H.K., Sharma K., Gupta J.K., Soni S.K. Production of a thermostable α-amylase from Bacillus sp. PS-7 by solid state fermentation and its synergistic use in the hydrolysis of malt starch for alcohol production. Process Biochem. 2005;40:525–534. [Google Scholar]
- 26.Van der Maarel M.J., Van der Veen B., Uitdehaag J.C., Leemhuis H., Dijkhuizen L. Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol. 2002;94:137–155. doi: 10.1016/s0168-1656(01)00407-2. [DOI] [PubMed] [Google Scholar]
- 27.Volkin D.B., Klibanov A.M. Thermal destruction processes in proteins involving cysteine. J. Biol. Chem. 1989;262:2945–2950. [PubMed] [Google Scholar]


