Abstract
Non-tuberculous mycobacteria isolated at the Central Public Health Laboratory from Mato Grosso do Sul in 2003 and 2004 were identified by conventional phenotypic methods (TI) and by PCR-Restriction Enzyme Analysis (PRA) using the hsp65 gene as target (PRA-hsp65). With 15 of the 32 analysed isolates, results of both methods were concordant, being 8 Mycobacterium avium, 3 M. fortutium, 1 M. kansasii, 1 M. flavescens, 1 M. peregrinum and 1 Nocardia brasiliensis. TI of 12 isolates was inconclusive. Novel PRA-hsp65 patterns were observed with 11 isolates. Medical data were evaluated for inference of clinical relevance of these isolates.
Keywords: Mycobacterium, PCR, Identification
Mycobacteria other than Mycobacterium tuberculosis (MOTT), also collectively called non-tuberculous mycobacteria (NTM), were described more than 50 years ago. They have been isolated from pulmonary, lymph node, skin, soft tissue, skeletal, and disseminated infections as well as during nosocomial outbreaks related to inadequate disinfection/sterilization of medical devices (9,13). NTM are acid-fast bacilli (AFB), morphologically similar to tuberculosis bacilli, and are ubiquitously distributed in the environment. Isolation of such bacteria from clinical specimens can represent either colonization or disease.
Isolates from sterile sites should be always considered as indicative of disease, but the clinical significance of isolates from non-sterile sites depends on the association of relevant clinical and epidemiological data (3). The American Thoracic Society recommends that NTM should be cultured from two separate expectorated sputum samples or at least from one bronchial wash or lavage to corroborate the diagnosis of NTM pulmonary disease in patients with pulmonary symptoms or suggestive images on chest radiograph (10). In Brazil, a publication from Centro de Vigilância Epidemiológica “Alexandre Vranjac” and Institute Adolfo Lutz from São Paulo classified the clinical significance of isolates in suggestive, potentially suggestive, and rarely suggestive of disease, according to microbiological criteria, as follows (4):
| Disease | Mycobacterial species | Type of specimen | Number of specimens |
|---|---|---|---|
| Suggestive | Potentially pathogenic | Sterile site | one |
| Non-sterile site | At least three | ||
| Potentially suggestive | Potentially pathogenic | Non-sterile site | two |
| Rarely suggestive | Rare pathogen | Non-sterile site | One or two |
NTM have become relevant after the beginning of the Aids pandemic (9). The morbidity and mortality impact of NTM in HIV-positive patients stimulated studies on epidemiology, ecology, genetics and molecular biology of mycobacteria. The recent increase in occurrence of mycobacterioses also led to the development of rapid identification methods, reducing time for diagnosis and for institution of specific treatment, increasing chances of therapeutic success (7).
Studies on the morbidity and mortality impact of mycobacterioses in Brazil are scarce and there is no report on the occurrence these infections in the State of Mato Grosso do Sul (2). The Central Public Health Laboratory from Mato Grosso do Sul is the reference laboratory for tuberculosis diagnosis and receives specimens from 77 cities of Mato Grosso do Sul State for bacilloscopy and culture. Identification of mycobacteria is not routinely performed.
Here we report a descriptive retrospective study of all AFB positive samples received by this laboratory in the period of January 2003 to May 2004 and the analysis of clinical and epidemiological data from patients with NTM positive cultures. This project was approved by the Ethics Committee from the Federal University of Mato Grosso do Sul number 364.
From 2,923 clinical samples received in the study period, 267 presented AFB smear positive results. These specimens were decontaminated by the Petroff method and cultivated on solid Löwenstein-Jensen (L-J) or Ogawa-Kudoh media, and in liquid Middlebrook 7H9 medium using the MGIT system (Mycobacteria Growth Indicator Tube, Beckton Dickinson, USA). Presence of AFB in positive cultures was confirmed by bacilloscopy after Ziehl-Neelsen staining. One hundred and fifty-one positive cultures were presumptively identified as M. tuberculosis by analysis of colony morphology (rough) and pigmentation (nonchromogen), and the presence of cord on bacilloscopy (14). Forty additional cultures did not present these characteristics at the first examination and were regarded as NTM. They were identified by traditional methods, which included analysis of phenotypic properties (growth rate, pigment production, growth in different temperatures) and results of biochemical tests (nitrate reduction, catalase activity, urease activity, tween 80 hydrolysis, arylsulfatase) at the Central Laboratory of Instituto Adolfo Lutz from São Paulo (6).
Molecular identification was performed concomitantly, by a different team, using the method of PCR-Restriction Enzyme Analysis (PRA) with the hsp65 gene as target. DNA from all isolates was obtained from a loop full of bacteria from solid plates, resuspended in ultra pure water and subjected to three freezing and boiling cycles of ten minutes each. A 441 bp fragment of the hsp65 gene was amplified by PCR with primers Tb11 (5’- ACCAACGATGGTGTGTCCAT) and Tb12 (5’-CTTGTCG AACCGCATACCCT) and subjected to enzymatic restriction with BstE II and Hae III, as described by Telenti et al. (17). Enzymatic digestion products were visualized after electrophoresis in 4% agarose gels (FMC, Biologia Molecular do Brasil) stained with ethidium bromide. Images were captured in Polaroid films and restriction patterns were analyzed using the GelCompar II program v. 2.5 (AppliedMaths, Sint-Marten Latten, Belgium). Calculated restriction fragment sizes were compared to published patterns (13,15) and to the patterns reported on the PRAsite (http://app.chuv.ch/prasite).
Eight of these 40 isolates were identified as M. tuberculosis by both identification methods and were excluded from this study. Considering the remaining 32 positive cultures, obtained from 28 patients, traditional identification assigned 17 (53.1%) isolates a mycobacterial species or complex, one culture was presumptively identified as Nocardia, 12 cultures presented ambiguous phenotypic results and were classified in groups according to growth rate and pigmentation, and two cultures were contaminated (Table 1).
Identification results of AFB isolates analyzed in this work and patients’ demographic data.
| No | PATIENT | ORIGIN | PRA-hsp65 | TI | SPECIMEN | HIV |
|---|---|---|---|---|---|---|
| 1 | A | Campo Grande | M. avium 1 | MAC | bone marrow | P |
| 2 | B | Corumbá | M. avium 1 | MAC | Sputum | N |
| 3 | C | Campo Grande | M. avium 1 | MAC | bone marrow | P |
| 4 | D | Campo Grande | M. avium 2 | MAC | Sputum | N |
| 5 | E | Campo Grande | M. avium 2 | MAC | Sputum | N |
| 6 | F | (indigenous) | M. avium 2 | MAC | Sputum | N |
| 7 | G | Campo Grande | M. avium 2 | MAC | Faeces | P |
| 8 | H | Corumbá | M. fortuitum 1 | M. fortuitum | bronchial lavage | N |
| 9 | I | (indigenous) | M. fortuitum 1 | M. fortuitum | Sputum | N |
| 10 | J | Corumbá | M. fortuitum 1 | M. fortuitum | Sputum | NA |
| 11 | K | Angélica | M. kansasii 5 | M. kansasii | Sputum | N |
| 12 | L | Itaporã | M. flavescens 1 | M. flavescens | Sputum | N |
| 13 | M | Rio Negro | M. peregrinum 2 | M. peregrinum | Sputum | N |
| 14 | N | Campo Grande | N. brasiliensis | Nocardia | Sputum | N |
| 15 | O | Aquidauana | * | MAC | Blood | P |
| 16 | M. peregrinum 2 | MAC | bone marrow | P | ||
| 17 | P | Miranda | M. peregrinum 2 | contaminated | Sputum | N |
| 18 | Q | (indigenous) | M. lentiflavum 3 | SGE | Sputum | N |
| 19 | R | (indigenous) | M. lentiflavum 2 | RGE | Sputum | N |
| 20 | S | Pedro Gomes | M. vaccae 1 | RGE | bone marrow | N |
| 21 | T | Campo Grande | mixed | MAC | Sputum | N |
| 22 | U | Corumbá | NP1 | SGA | Sputum | P |
| 23 | NP1 | SGA | Sputum | P | ||
| 24 | V | Corumbá | NP2 | RGA | Sputum | N |
| 25 | NP2 | RGE | Sputum | N | ||
| 26 | W | Rio Negro | NP3 | SGE | Sputum | N |
| 27 | NP4 | RGE | Sputum | N | ||
| 28 | X | Aquidauana | NP5 | M. gordonae | Sputum | N |
| 29 | Y | (indigenous) | NP6 | contaminated | Sputum | N |
| 30 | Z | Campo Grande | NP7 | SGE | Sputum | NA |
| 31 | AA | Campo Grande | NP8 | RGE | Urine | N |
| 32 | AB | (indigenous) | NP9 | RGA | Sputum | N |
No = isolate number; PRA-hsp65 = molecular identification by PCR-Restriction Enzyme Analysis with the hsp65 gene as target; NP = novel PRA-hsp65 pattern; TI = traditional identification by phenotypic analysis and biochemical tests; HIV = patient HIV status: P = positive, N = negative, NA = not available; MAC = M. avium complex. Isolates with concordant identification by both methods are in bold. Indigenous patients did not have origin information.
PRA-hsp65 pattern shared by seven species, among them two SGA: M. avium 3 and M. intracellulare 3, RGA = nonchromogenic rapid grower, RGE = scotochromogenic rapid grower, SGA = nonchromogenic slow grower, SGE = scotochromogenic slow grower.
PRA-hsp65 assigned 19 (59.4%) NTM cultures a mycobacterial species. Interestingly, culture 14 has been identified as Nocardia sp. by traditional identification and the obtained PRA-hsp65 pattern was recently assigned to Nocardia brasiliensis (5). This confirms that PRA-hsp65 could be also useful for identification of species of Nocardia. PRA restriction patterns from isolate 20 were suggestive of mixed culture but the original culture could not be recovered for further studies. The remaining eleven cultures were distributed in nine novel PRA-hsp65 patterns, not present in PRA databases (Table 1, Fig. 1).
Figure 1.
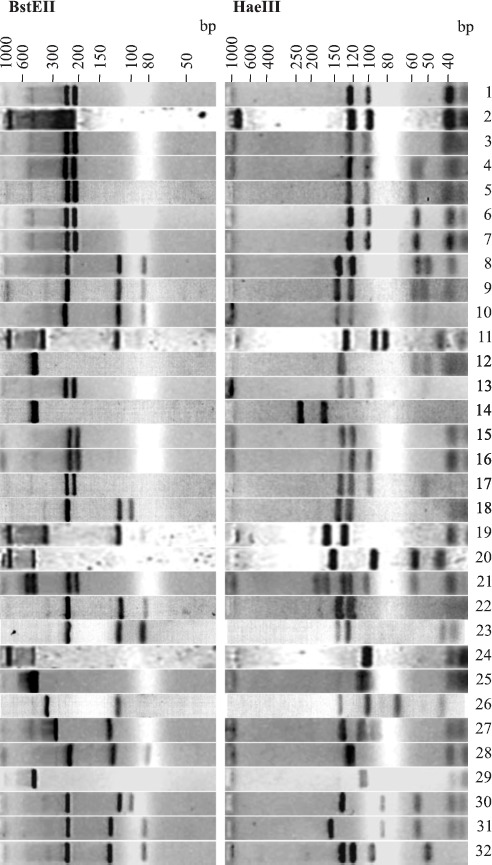
PRA-hsp65 patterns of 32 isolates analyzed using GelCompar II version 2.5.
In 15 (46.8%) cultures, traditional identification and PRA-hsp65 achieved concordant results for a mycobacterial species and this identification was regarded as correct.
Isolate 16 was identified as M. avium complex (MAC) by traditional identification and M. peregrinum 2 by PRA-hsp65. Laboratory contamination during manipulation of this isolate could not be ruled out, especially as other isolates identified as M. peregrinum 2 were obtained in this study. Isolate 15, from the same patient, was also identified as MAC and PRA-hsp65 resulted in a pattern of BstE II [235 bp-210 bp] and Hae III [145 bp-130 bp], which is shared by seven different species (see Figure 1 and the PRAsite). Two of these seven species are nonchromogenic slow-growers, M. avium 3 and M. intracellulare 3, which would be in concordance with the phenotypic identification of MAC.
Species identification obtained by PRA-hsp65 from isolates 17 to 20 could not be confirmed by traditional identification. Two of these isolates, 18 and 19, obtained from sputum from two indigenous patients, were identified by PRA-hsp65 as M. lentiflavum, a species recently identified in Brazilian clinical isolates (16). M. lentiflavum is difficult to identify by conventional methods and the prevalence and clinical significance of such species in Brazil has yet to be fully evaluated.
Culture 28 was identified as M. gordonae by traditional identification, but the observed PRA-hsp65 pattern was not present in available databases. More than ten PRA-hsp65 patterns have been described for M. gordonae and it is considered a highly polymorphic species with respect to PRA-hsp65 patterns (12).
Identification by both methods was inconclusive for the remaining ten isolates.
In a similar study performed with isolates from São Paulo State, concordant results of traditional identification and PRA-hsp65 were obtained in 76 (73.8 %) out of 103 isolates, discordant and inconclusive PRA and/or biochemical results were obtained from 27 (26.2%) isolates (7). These presence of ten (31.2%) isolates without identification by both methods in the present work suggests the existence of not yet described mycobacterial species in the geographic region studied here, and could explain the lower percentage of conclusive results obtained here.
Medical data from all patients with NTM cultures, with exception of patient Z, were analyzed. Nine patients (A-G, O, and T) had cultures identified as M. avium by PRA-hsp65 and/ or MAC by traditional identification. Four cultures were isolated from sterile sites from three HIV-positive patients and, according to criteria described above, the diagnosis of NTM disease could have been confirmed right away in these cases (10). M. avium was also isolated from feaces (one sample) from a fourth HIV- positive patient. These four patients (A, C, G, and O) received regular treatment for tuberculosis and died during the study. No information about treatment or outcome was available in the clinical files from other patients infected with M. avium.
Isolates identified as M. fortuitum, M. peregrinum, M. kansasii, M. lentiflavum, and M.flavescens were isolated once from sputum. Since these species are potentially pathogenic, the analysis of additional specimens would be required for confirmation of NTM disease (4). According to medical data, no specific antimicrobial treatment was given to these patients.
Isolate 20 was identified as M. vaccae 1 by PRA-hsp65 and was isolated from a sterile site (bone marrow). This species is rarely described as a cause of human disease, but cutaneous and pulmonary infections have been reported in USA (11). This patient improved after specific treatment for leishmaniasis and the role of M. vaccae in the clinical picture could not be confirmed.
Cultures 22 and 23, with identical identification results, were obtained from the same patient with one-week interval. The species could not be determined. This HIV-positive patient had symptoms of pulmonary disease (productive cough, thoracic pain and fever), therefore diagnosis of NTM pulmonary disease could have been confirmed here, but the medical file lacks reference about treatment of this patient.
Isolates 24 and 25 were obtained from the same patient, who had pulmonary symptoms and was treated for tuberculosis and eventually for multi-drug resistant tuberculosis (MDR-TB) for 12 months with no improvement. Both isolates showed the same PRA-hsp65 pattern but the species could not be identified. In this case also, diagnostic criteria for confirmation of NTM pulmonary disease were fulfilled (10).
Cultures 26 and 27 were obtained from the same patient with one-year interval. This patient was treated for tuberculosis since the first isolate and the disease reactivated after one year. The isolates comprised different mycobacteria, which could not be identified to the species level. The occurrence of two different infections could not be ruled out nor fully confirmed in this case.
This was a retrospective study, but it revealed that eight (20%) from 40 cultures presumptively identified as NTM by macroscopic and microscopic culture characteristics contained M. tuberculosis. In addition, at least six (21.4%) out of 28 patients with NTM positive cultures received unnecessary treatment for tuberculosis and even for MDR-TB, probably justified by the presence of AFB smear positive results. Full identification of cultured specimens could have confirmed or excluded tuberculosis and helped to re-direct the therapeutic approach in due time, provided that the proposed diagnostic criteria were observed to confirm disease and exclude specimen contamination (4,10).
Better management of patients with suspected NTM infections is mandatory and could be obtained by local implementation of rapid methods for identification of mycobacteria. PRA-hsp65 is useful and reliable for separation of M. tuberculosis from NTM. Compared to traditional identification, it is quicker (one week versus one to two months), poses less contamination risks, and can be performed in laboratories other than reference centers. Also, reagents’ costs are lower in comparison to traditional identification. Other techniques, not performed in the present study, would be useful and should be performed in clinically relevant cases to resolve discordant or inconclusive identification results, specially DNA sequencing of conserved regions, as 16S rDNA, rpoB, and hsp65 genes (1,8).
RESUMO
Identificação de micobactérias não-tuberculosas do Laboratório Central de Saúde Pública de Mato Grosso de Sul e análise de dados clínicos dos pacientes
Micobactérias não-tuberculosas isoladas no Laboratório Central de Saúde Pública de Mato Grosso do Sul em 2003 e 2004 foram identificadas usando métodos fenotípicos convencionais (TI) e PCR-Restriction Enzyme Analysis (PRA) tendo o gene hsp65 como alvo (PRA-hsp65). Em 15 dos 32 isolados analisados os resultados obtidos com ambos métodos foram concordantes, sendo 8 Mycobacterium avium, 3 M. fortutium, 1 M. kansasii, 1 M. flavescens, 1 M. peregrinum e 1 Nocardia brasiliensis. TI de 12 isolados não foi conclusiva. Perfis não descritos de PRA- hsp65 foram observados com 11 isolados. Dados dos prontuários médicos foram avaliados para inferir a relevência clínica dos isolados.
Palavras-chave: Micobactérias, PCR, Identificação.
REFERENCES
- 1.Adekambi T., Drancourt M. Dissection of phylogenetic relationships among 19 rapidly growing Mycobacterium species by 16S rRNA, hsp65, sodA, recA and rpoB gene sequencing. Int. J. Syst. Evol. Microbiol. 2004;54:2095–2105. doi: 10.1099/ijs.0.63094-0. (Pt 6) [DOI] [PubMed] [Google Scholar]
- 2.Barreto A.M.W., Campos C.E.D. Micobacterias nao- tuberculosas no Brasil. Bol. Pneum. Sanit. 2000;8(1):23–32. [Google Scholar]
- 3.Campos H.S. Manejo da doença micobacteriana não tuberculosa. In: Boi. de Pneumologia SanitÀria., editor. FUNASA/ CENEPI/CNPS/CRPHF, Rio de Janeiro; 2000. pp. 39–50. [Google Scholar]
- 4.Centro de Vigilancia Epidemiológica “Prof. Alexandre Vranjac” – Divisão de Tuberculose, Instituto Adolfo Lutz – Setor de Micobactérias Micobacteriose: recomendares para o diagnóstico e tratamento. 2005. Secretaria Estadual da Saúde de São Paulo ftp://ftp.cve.saude.sp.gov.br/doc_tec/tb/MNT_Final_9-12-05a.pdf.
- 5.Chang P.L., Hsieh W.S., Chiang C.L., Tuohy M.J., Hall G.S., Procop G.W., Chang H.T., Ho H.T. The hsp65 gene patterns of less common Mycobacterium and Nocardia spp. by polymerase chain reaction-restriction fragment length polymorphism analysis with capillary electrophoresis. Diagn Microbiol. Infect. Dis. 2007;58(3):315–323. doi: 10.1016/j.diagmicrobio.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Collins C.H., Grange J.M., Yates M.D. Tuberculosis Bacteriology: Organization and Practice. Oxford: Butterworth-Heinemann; 1997. [Google Scholar]
- 7.da Silva C.F., Ueki S.Y., Geiger D.C., Leao S.C. hsp65 PCR-restriction enzyme analysis (PRA) for identification of mycobacteria in the clinical laboratory. Rev. Inst. Med. Trop. São Paulo. 2001;43(1):25–28. doi: 10.1590/s0036-46652001000100005. [DOI] [PubMed] [Google Scholar]
- 8.Devulder G., Perouse de Montclos M., Flandrois J.P. A multigene approach to phylogenetic analysis using the genus Mycobacterium as a model. Int. J. Syst. Evol. Microbiol. 2005;55:293–302. doi: 10.1099/ijs.0.63222-0. (Pt 1) [DOI] [PubMed] [Google Scholar]
- 9.Falkinham J.O. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 1996;9(2):177–215. doi: 10.1128/cmr.9.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith D.E., Aksamit T., Brown-Elliott B.A., Catanzaro A., Daley C., Gordin F., Holland S.M., Horsburgh R., Huitt G., Iademarco M.F., Iseman M., Olivier K., Ruoss S., von Reyn C.F., Wallace R.J., Jr., Winthrop K. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 11.Hachem R., Raad I., Rolston K.V., Whimbey E., Katz R., Tarrand J., Libshitz H. Cutaneous and pulmonary infections caused by Mycobacterium vaccae. Clin. Infect. Dis. 1996;23(1):173–175. doi: 10.1093/clinids/23.1.173. [DOI] [PubMed] [Google Scholar]
- 12.Hafner B., Haag H., Geiss H.K., Nolte O. Different molecular methods for the identification of rarely isolated non-tuberculous mycobacteria and description of new hsp65 restriction fragment length polymorphism patterns. Mol. Cell Probes. 2004;18(1):59–65. doi: 10.1016/j.mcp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Leao S.C., Martin A., Mejia G.I., Palomino J.C., Robledo J., Telles M.A.S., Portaels F. Practical handbook for the phenotypic and genotypic identification of mycobacteria. Brugges: Vanden BROELLE; 2004. [Google Scholar]
- 14.Monteiro P.H.T., Martins M.C., Ueki S.Y.M., Giampaglia C.M.S., Telles M.A.S. Cord formation and colony morphology for the presumptive identification of Mycobacterium tuberculosis complex. Braz. J. Microbiol. 2003;34(2):171–174. [Google Scholar]
- 15.Rastogi N. An introduction to bacterial taxonomy, structure, drug resistance, and pathogenesis. In: Dioniosio D., editor. Textbook- atlas of intestinal infections in AIDS. Milano, Italia: Springer-Verlag; 2003. pp. 89–115. [Google Scholar]
- 16.Suffys P, Rocha A.S., Brandao A., Vanderborght B., Mijs W., Jannes G., Mello F.C., Pedro H.S., Fonseca L.S., de Oliveira R.S., Leao S.C., Saad M.H. Detection of mixed infections with Mycobacterium lentiflavum and Mycobacterium avium by molecular genotyping methods. J. Med. Microbiol. 2006;55:127–131. doi: 10.1099/jmm.0.46218-0. (Pt 1) [DOI] [PubMed] [Google Scholar]
- 17.Telenti A., Marchesi F., Balz M., Bally F., Bottger E.C., Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J. Clin. Microbiol. 1993;31(2):175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


