Abstract
Staphylococci bacteria are involved in many human and animal infections and development of alternative antimicrobial drugs against pathogenic bacteria is of great interest to the pharmaceutical industry. This study investigated the in vitro effect of Rauvolfia grandiflora methanol extract (root bark fraction) (RGE) on the density of ATCC strains of Staphylococcus aureus and Staphylococcus epidermidis, and a clinical enterotoxin-producer, S. aureus bovine strain. The alkaloid, isoreserpiline, obtained from dichloromethane extract of R. grandiflora was ineffective against the strains tested. After incubation of staphylococci strains in the presence of 1.2 μg.mL-1 RGE, a significant inhibition of cell growth was observed using both spectrophotometry and ELISA assays. Twelve drugs were evaluated for their antimicrobial effects on culture RGE-treated cells using the disk diffusion method. Penicillin resistant strains became sensitive to the drug after RGE treatment. Furthermore, enterotoxin production by RGE-treated S. aureus was evaluated using a standardized ELISA method. Although staphylococcal LSA 88 bovine strain cells remained viable after exposure to the extract, enterotoxin production was precluded in 20% after RGE treatment. Significant interference in staphylococci cell density, drug sensitivity and enterotoxin secretion was observed after treatment. The study highlights the necessity to find new methods of disease prevention and new antibiotic therapies against staphylococcal infections.
Keywords: Rauvolfia grandiflora, Staphylococcus aureus, enterotoxin, antimicrobial activity.
Submitted: August 07, 2009; Returned to authors for corrections: September 04, 2009; Approved: March 16, 2010.
INTRODUCTION
Staphylococcus aureus is a human pathogen encapsulated bacterium with anti-phagocytic activity (26,15,30) which can invade and survive within a wide variety of mammalian cells (2,14) and causes intramammary infections in animals (27,34). The detection of a major S. aureus clone A (63%), infecting dairy herds, has demonstrated its geographical spread among farms in Rio de Janeiro State, Brazil, affecting five different animal species and it has been suggested that all but one of the clones was animal specific (1,34). S. aureus causes food poisoning epidemics in humans (5,24) by secreting staphylococcal enterotoxins (SEs). However, their role in intoxications has yet to be clarified, and descriptions of the bacterial pathogenicity continues to grow as more proteins with the same or similar properties are isolated or characterized (20,16,18,25,31). SEs are known to function as potent super-antigens that stimulate non-specific clonal T-cell proliferation, followed by a state of hyporesponsiveness to subsequent antigen stimulation and, thus, hinder the development of protective immunity, while promoting the persistence of bacteria in the host (11). SEs may also aggravate bovine intramammary infections throughout local cytokine release (40). SEs are produced by S. aureus after agr activation quorum-sensing system (QS), which consists of a sophisticated system to ensure that some bacteria functions only take place when a specific population density has been reached (3). This agr commanded system, however, is negatively influenced by the octapeptide RNAIII Inhibiting Peptide (RIP) in vivo by interfering in staphylococcal infections (4) and in vitro by polyclonal affinity purified antibodies anti-recTRAP, resulting in staphylococcal enterotoxins inhibition in liquid culture (35). Several plant extracts containing phenolics and alkaloid compounds may interfere with bacterial (QS) and biofilm formation (13,29).
As a group, the coagulase-negative staphylococci are among the most frequently-isolated bacteria in clinical microbiology and are becoming increasingly important, especially as causes of hospital-acquired infections, including S. epidermidis (37), considered a normal inhabitant of human skin and mucous membranes, but they also infect animals (32,38).
Several plant-derived compounds possess medicinal properties with activity against important medical microorganisms (17,29). A new indole alkaloid isolated from root bark was described in Rauvolfia grandiflora, however, its biological activity has not as yet been tested (7,8). Previous studies have shown that plant extracts can inhibit enterotoxin production by Staphylococcus aureus strains (22,6). A natural product found in the bark of witch hazel, hamamelitannin, had no effect on staphylococcal growth in vitro, but inhibited the (QS) regulator RNAIII and, in a rat graft model, prevented device-associated infections in vivo, including infections caused by MRSA and MRSE strains (19). The present study describes the biological activity of methanol extract from Rauvolfia grandiflora against staphylococci strains, and discusses the interference of enterotoxin production by S. aureus, cell growth inhibition and antibiotic interactions with the bacteria.
MATERIAL AND METHODS
Test organisms and plant extract
Root bark of R. grandiflora was collected in São Francisco do Itabapoana City, Rio de Janeiro State, Brazil. A voucher specimen was deposited in the herbarium of the Agricultural University of wageningem, Netherlands. Root bark samples were dried, powdered, and extracted using methanol and after solvent evaporation, all samples were dissolved in DMSO (Sigma, USA) at a concentration of 100000 μg.mL-1. The assays were performed using the extracts at a final concentration of 1.2 μg.mL-1 at RT.
Reference bacterial strains: S. aureus Wood 46 (ATCC10832) a capsule-negative strain, S. aureus Smith Diffuse (SD) (ATCC 13709) a capsule-producer strain, S. aureus ATCC 25923 strain, and S. epidermidis ATCC 12228 a non-biofilm-forming strain. Bovine S. aureus LSA88 (34) is a SEC/SED producing strain previously studied (35,36) and used in the development of the recombinant sec gene (33). All strains were re-activated in Brain Heart Infusion broth.
Fractionation of RGE
The methodology used to obtain RGE followed a previously alkaloid extraction from R. grandiflora with minor modifications (8). Dried and powdered root bark (1.67 kg) from R. grandiflora was extracted with methanol at room temperature, resulting in 36.0g of residue, after solvent evaporation. About 95% of this residue was chromatographed on a silica gel column eluted with a methanol gradient in dichloromethane, and 10 fractions collected. Nine of these samples were re-fractionated but the concentrations were insufficient to carry out the tests. The isolated alkaloid compound isoreserpiline, obtained from dichloromethane fraction, was tested against all strains.
Experimental procedures
Growth Inhibition: An aliquot of 0.1 mL from Rauvolfia grandiflora root bark methanol extraction (RGE) was added to 1.8 mL of BHI and 0.1 mL of bacteria inoculum (OD550nm 0.5 McFarland), determined using a photometer (Densimat, bioMérieux, France). The density readings of treatments and controls were recorded every sixty minutes. Controls employed medium and inoculums with DMSO added as a dispersing solvent. After 12 h incubation period at 37°C, 0.1 mL aliquots of cultures were streaked on BHA agar (Acumedia, USA) and incubated on 37°C.
Antibiotic activities after extract treatments
Antibiograms were performed following exposure of strains to RGE. Pure colonies of each strain were sub-cultured on blood agar plates for antimicrobial standard disk diffusion tests, performed in agar Muller Hinton, according to the guidelines of the National Committee for Clinical Laboratory Standards (21), and the halo formed around each disk was recorded. The disks contained the following drugs: penicillin G (PEN, 10U), oxacillin (OXA, 1 μg), amoxicillin (AMO, 10 μg), ampicilin (AMP, 10 μg), cephalothin (CFL, 30 μg), cefoxitin (CFO, 30 μg), trimethoprim-sulfamethoxazole (SUT, 25 μg), clindamycin (CLI, 2 μg), erythromycin (ERI, 15 μg), gentamicin (GEN, 10 μg), tetracycline (TET 30 μg), and vancomicin (VAN, 30 μg) (Laborclin, PR, Brazil).
Growth and Enterotoxin inhibition assays
In order to evaluate the inhibition of SEC and SED secretion by LSA 88 bovine strain after treatment with RGE, ELISA assays were performed as previously described (35). After ON reactivation at 37°C, cells were harvested by centrifugation at 12 000 x g and washed twice in PBS. Cells were then diluted in fresh TECRA® Staphylococcal growth medium (Bioenterprises Pty. Ltd., Australia) to a density of 108 cells mL-1 at OD550nm 0.5. Tubes with cells plus DMSO were used as negative controls. Kit-positive and -negative controls were used. A proportional cell growth assay was conducted over a 2h duration, and growth of cells was interrupted by adding a stop solution. A second experiment was conducted, where S. aureus cell growth was monitored using a controlled incubation at 37°C for 3h for controls and 4h for treatments in order to standardize the values for cell growth and subsequent comparison of enterotoxin production. After centrifugation, supernatants were harvested and tested for SEs using the TECRA® Visual Immunoassay VIA? kit (Bioenterprises Pty. Ltd., Australia), according to the manufacturer’s instructions. This assay allows for the specific and sensitive detection of enterotoxins A to E (lower limit 1.0 ng.mL-1). Color development readings (OD405nm) (Biorad, USA) were realized during two intervals at the end of the assay. All the experiments described were performed in triplicate.
RESULTS
Following purification of RGE, the only alkaloid compound obtained, isoreserpiline, when tested alone against the bacterial strains selected here, showed no antimicrobial activity. The structure of isoreserpiline is shown in Figure 1. The other 9 fractions resulted in very low concentrations of the alkaloids darcyribeirine and β-yohimbine obtained from methanol extract and were not tested. ELISA results, as expected, showed that RGE-treated S. aureus LSA88 (bovine strain) cells were inhibited and directly accompanied by a proportional decreased enterotoxin production (Figure 2), i.e. the lower the cell density, the lower the amount of enterotoxin detected. RGE also caused growth inhibition of all other strains when cultured on BHA agar (Tukey, p<0.05%). Controls exhibited CFU counts above 300 colonies/mL, while RGE-treated cells presented CFU counts that were less than 300 and above 30 CFU.
Figure 1.
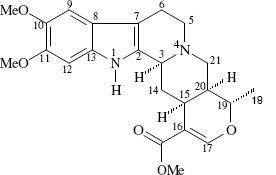
Structure of isoreserpiline obtained from dichloromethane fraction.
Figure 2.
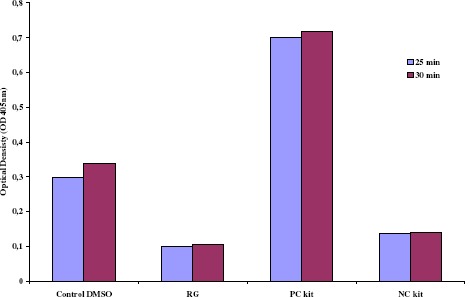
Density of Staphylococcus aureus (bovine strain) cells. Color development intervals (25 and 30 minutes readings). Production of enterotoxins by LSA 88 Staphylococcus aureus (bovine strain) was directly dependent/proportional on cell population. RG (treatment with Rauvolfia grandiflora extract); PC = positive control; NC= negative control.
ELISA experiments to determine enterotoxin production after RGE treatment revealed 20% inhibition in SE production, compared to controls, even with similar cell populations (Figure 3).
Figure 3.
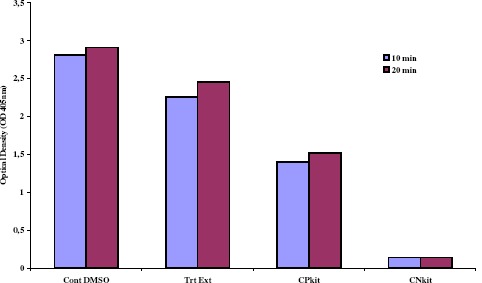
Color development intervals (10 and 20 minutes readings). Inhibition of enterotoxins produced by LSA 88 Staphylococcus aureus (bovine strain) cultured in liquid medium in the presence of R. grandiflora methanol extract, in proportional cell density (OD550nm) of both, treatment (Trt Ext incubated for 4h) and control (Cont DMSO incubated for 3h). PC and NC indicate positive and negative controls, respectively.
The results of antibiograms of RGE treated cells are shown in Tables 1A-2B.
Table 1A.
Antibiogram (measured in mm) of staphylococcal strains, treated with Rauvolfia grandiflora extract, and submitted to six drugs. Evaluated by agar diffusion method using Gram-positive A kit.
| Strains | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ATCC12228 | LSA88 | ATCC25923 | Wood46 | Smith Diffuse | ||||||
| GrA | Treat | Cont | Treat | Cont | Treat | Cont | Treat | Cont | Treat | Cont |
| PEN | 24.0Ad | 15.0Bc | 23.7Ac | 15.7Bd | 36.3Aa | 21.3Bb | 16.7Ad | 14.7Bd | 24.3Ae | 23.3Ad |
| CFL | 40.0Aa | 32.3Ba | 32.3Aa | 32.0Aa | 33.Ab | 26.3Ba | 34.0Aa | 29.7Ba | 38.0Aa | 37.0Aa |
| CLI | 40.0Aa | 33.0Ba | 27.0Ab | 20.7Bb | 28.3Ad | 19.7Bc | 31.7Ab | 27.3Bb | 25.3Ade | 25.3Ac |
| AMO | 28.3Ac | 15.0Bc | 24.3Ac | 14.7Bd | 31.3Abc | 22.3Bb | 17.0Ad | 14.7Bd | 36.7Ab | 34.3Bb |
| TET | 19.3Ae | 11.3Bd | 18.0Ad | 12.3Be | 29.7Acd | 25.7Ba | 16.3Ad | 13.7Bd | 26.3Ad | 25.7Ac |
| OXA | 36.3Ab | 26.3Bb | 23.7Ac | 18.3Bc | 25.0Ae | 21.7Bb | 21.0Bc | 25.3Ac | 28.0Ac | 26.0Bc |
GrA- Kit used for antibiogram to analyze six anti-Gram-positive A drugs; Means followed by the same small letter in the column (drug) and capital letter in the line (extract treatment), do not differ among themselves. Tukey test, 5% probability.
Table 2B.
Comparison of inhibition (measured in mm), among staphylococcal strains, treated by Rauvolfia grandiflora extract, and submitted to antibiogram towards six drugs by agar diffusion method using kit Gram-positive B.
| Drugs | VAN | AMP | ERI | SUT | GEN | CFO | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Treat | Cont | Treat | Cont | Treat | Cont | Treat | Cont | Treat | Cont | Treat | Cont |
| ATCC12228 | 27.7Aa | 21.3Ba | 23.7Ac | 13.7Bd | 31.3Aa | 23.7Ba | 34.3Aa | 22.7Bab | 33.0Aa | 24.0Ba | 45.3Aa | 41.0Ba |
| LSA88 | 20.0Abc | 14.7Bb | 31.7Ab | 23.7Bc | 21.7Ac | 13.3Bd | 28.3Ab | 17.7Bc | 23.3Abc | 17.0Bcd | 29.7Ad | 26.7Bd |
| ATCC25923 | 20.7Ab | 14.7Bb | 37.0Ba | 40.0Aa | 25.7Ab | 20.3Bb | 32.0Aa | 20.7Bbc | 25.0Ab | 18.7Bc | 34.0Bc | 37.7Ab |
| Wood46 | 18.7Ac | 14.7Bb | 15.7Ad | 14.3Ad | 22.0Ac | 17.7Bc | 28.3Ab | 23.0Bab | 21.3Ac | 15.7Bd | 37.3Ab | 35.3Bb |
| Smith Diffuse | 16.7Ad | 16.0Ab | 39.3Aa | 35.7Ab | 17.7Ad | 16.7Ac | 25.7Ab | 24.0Ba | 22.0Ac | 21.0Ab | 32.7Acd | 31.7Ac |
GrB- Kit used for antibiogram to analyze six drugs anti-Gram-positive B; Media followed by the same small letter in the column (strains) and capital letter in the line (extract treatment), do not differ among themselves. Tukey test, 5% probability.
Individually, after antibiotic exposure of bacteria, comparing RGE treated cells and controls, the results show larger inhibition zones than those of the respective controls for most strains (p<0.05). In contrast, S. aureus 25923 and Wood 46 strains, did not present larger inhibition zones after exposure to oxacillin (Table 1A) and ampicillin (Table 1B), respectively, suggesting no effect of the treatment. For SD strain, treatment did not affect the activity of drugs, except for amoxicillin, oxacillin, and trimethoprim-sulfamethoxazole, where RGE augmented the inhibition zones. Comparing results between drugs, cephalosporin (CFL and CFO) demonstrated greater activity than the other drugs against most of the strains tested. Tables 2A and 2B show that the SD strain remained less sensitive to drugs, except for OXA and SUT. All other strains were responsive to RGE treatment and exhibited higher sensitivity towards the drugs tested, as confirmed by the augmentation of the inhibition zone (p<0.05).
Table 1B.
Antibiogram (measured in mm) of staphylococcal strains, treated by Rauvolfia grandiflora extract, and submitted to six drugs. Evaluated by agar diffusion method using Gram-positive B kit.
| Strains | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ATCC12228 | LSA88 | ATCC25923 | Wood46 | Smith Diffuse | ||||||
| GrB | Treat | Cont | Treat | Cont | Treat | Cont | Treat | Cont | Treat | Cont |
| VAN | 27.7Ad | 21.3Bb | 20.0Ad | 14.7Bd | 20.7Ad | 14.7Bc | 18.7Ad | 14.7Bd | 16.7Ae | 16.0Ae |
| AMP | 23.7Ae | 13.7Bc | 31.7Aa | 23.7Bb | 37.0Ba | 40.0Aa | 15.7Ae | 14.3Ad | 39.3Aa | 35.7Aa |
| ERI | 31.3Ac | 23.7Bb | 21.7Acd | 13.3Bd | 25.7Ac | 20.3Bb | 22.0Ac | 17.7Bc | 17.7Ae | 16.7Ae |
| SUT | 34.3Ab | 22.7Bb | 28.3Ab | 17.7Bc | 32.0Ab | 20.7Bb | 28.3Ab | 23.0Bb | 25.7Ac | 24.0Bc |
| GEN | 33.0Abc | 24.0Bb | 23.3Ac | 17.0Bc | 25.0Ac | 18.7Bb | 21.3Ac | 15.7Bcd | 22.0Ad | 21.0Ad |
| CFO | 45.3Aa | 41.0Ba | 29.7Aab | 26.7Ba | 34.0Aab | 37.7Ba | 37.3Aa | 35.3Ba | 32.7Ab | 31.7Ab |
GrB- Kit used for antibiogram to analyze six anti-Gram-positive B drugs; Means followed by the same small letter in the column (drug) and capital letter in the line (extract treatment), do not differ among themselves. Tukey test, 5% probability.
Table 2A.
Comparison of inhibition (measured in mm) of staphylococcal strains, treated by Rauvolfia grandiflora extract, and submitted to antibiogram towards six drugs by agar diffusion method using Gram-positive A kit.
| Drugs | PEN | CFL | CLI | AMO | TET | OXA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strains | Treat | Cont | Treat | Cont | Treat | Cont | Treat | Cont | Treat | Cont | Treat | Cont |
| ATCC12228 | 24.0Ab | 15.0Bc | 40.0Aa | 32.3Bb | 40.0Aa | 33.0Ba | 28.3Ac | 15.0Bc | 19.3Ac | 11.3Bc | 36.3Aa | 26.3Ba |
| LSA88 | 23.7Ab | 15.7Bc | 32.3Ac | 32.0Ab | 27.0Ac | 20.7Bd | 24.3Ad | 14.7Bc | 18.0Acd | 12.3Bbc | 23.7Ac | 18.3Bc |
| ATCC25923 | 36.3Aa | 21.3Bb | 33.0Ac | 26.3Bd | 28.3Ac | 19.7Bd | 31.3Ab | 22.3Bb | 29.7Aa | 25.7Ba | 25.0Ac | 21.7Bb |
| Wood46 | 16.7Ac | 14.7Bc | 34.0Ac | 29.7Bc | 31.7Ab | 27.3Bb | 17.0Ae | 14.7Bc | 16.3Ad | 13.7Bb | 21.0Bd | 25.3Aa |
| Smith Diffuse | 24.3Ab | 23.3Aa | 38.0Ab | 37.0Aa | 25.3Acd | 25.3Ac | 36.7Aa | 34.3Ba | 26.3Ab | 25.7Aa | 28.0Ab | 26.0Ba |
GrA- Kit used for antibiogram to analyze six anti-Gram-positive A drugs; Means followed by the same small letter in the column (strains) and capital letter in the line (extract treatment), do not differ among themselves. Tukey test, 5% probability.
DISCUSSION
Staphylococcus aureus and S. epidermidis may represent a threat to human health and animals by surpassing host immune response with an arsenal of virulence factors, most of them based on genetic regulation, i.e. quorum sensing (QS) (3,38). Many other pathogenic bacteria use the same regulation system to secrete their virulence factors, however, polyphenols such as flavonoids from plants, can affect biofilm formation by interfering with QS in B. cepacia (13). Recently, epigallocatechin gallate (EGCG) from green tea, was tested for its ability to inhibit QS in Chromobacterium violaceum, and even following an increase in bacteria growth due to the addition of EGCG, the production of QS-dependent violacein by the organism was lower (28). Other workers have observed that hamamelitannin, a natural product found in the bark of Hamamelis virginiana, had no effect on drug resistance and growth in vitro but inhibited the quorum-sensing regulator, RNAIII, of staphylococcal strains by interfering in the competition with RAP (19). Different products, including RNAIII inhibiting peptide (RIP) and anti-TRAP IgY polyclonal antibodies were able to inhibit the growth of S. aureus strains (35) as observed in the present work. Inhibition of enterotoxin production by S. aureus, at the QS level, as suggested herein, may be due to interference with surface receptors for RAP activation and with subsequent transcription of virulence genes, including SE genes as proposed previously (4). Here, enterotoxin production was precluded in 20% by treatment of LSA 88 (bovine strain) with RGE. The method used in the present study may represent real inhibitory activity on staphylococcal enterotoxin secretion, if one compares this to SE inhibition using an agglutination test (22), which may cause misinterpretation of the results. Also, enterotoxin A inhibition was assessed using a membrane over agar method and measured by immunodifusion assay (6), which is less sensitive than ELISA SEs screening used in the present work. Alkaloid-containing extracts from different plants also show antimicrobial activity, including activity against S. aureus (29,9). Although we have not totally identified the constituents of RGE, alkaloids are known to be produced by this plant (7,8). Apocynaceae are known to be alkaloid-producing plants, but the alkaloids vary in their chemical structures (7,8,29). The lack of activity of the purified compound, isoreserpiline, on the staphylococci tested may be explained by the interference of others compounds present in RGE (10) or did not present activity alone. The results of the present study, using low concentrations of RGE, showed significant growth inhibition on S. aureus strains. In order to investigate any variation among staphylococci from different origins, the antibiogram analysis showed that most of the RGE-treated strains were affected by exposure to drugs. However, in the case of Wood46 and ATCC 25923, where inhibition zones of (OXA) and (AMP), both beta-lactamic drugs, presented inverted results for treatments compared with all other strains. Although nearly all S. aureus strains are resistant to PEN (12), in the present study some resistant strains after RGE treatment became sensitive to the drug, demonstrating inhibition zones between 28 and 29 mm, for resistant and sensitive β-lactamase positive Staphylococcus, respectively (Adapted from CLSI, M100-S17, Jan 2007). The ATCC 12228 S. epidermidis strain, a MSSE (Methicillin Susceptible Staphylococcus epidermidis), remained susceptible to all drugs but with a larger halo after RGE treatment. Results suggest that differences in antibiotic zone inhibition of treated staphylococci growth could result from the increased sensitivity of S. aureus. This may occur in a QS-dependent manner by interfering in sae, a locus related to resistance and susceptibility towards drugs in the bacteria (23,39). Taken together the results reported in the present investigation may contribute to the understanding of plant extract activities in the strains tested, although further studies are needed.
ACKNOWLEDGEMENTS
The authors thank FAPERJ and CAPES for financial support and CNPq for grant and scholarships support. We also wish to thank Dr. Richard Ian Samuels (UENF) for reviewing the text.
REFERENCES
- 1.Aires-de-Sousa, M.; Parente, C.E.S.R.; Vieira-da-Motta, O.; Bonna, I.C.F.; Silva, D.A.; de Lencastre, H. (2007). Characterization of Staphylococcus aureus isolated from buffalo, bovine, ovine, and caprine milk samples from Rio de Janeiro State, Brazil. Appl. Environ. Microbiol. 73 (12), 3845–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida, R.A.; Matthews, K.R.; Cifrian, E.; Guidry, A.J.; Oliver, S.P. (1996). Staphylococcus aureus invasion of bovine mammary epithelial cells. J. Dairy Science 79, 1021–1026. [DOI] [PubMed] [Google Scholar]
- 3.Balaban, N.; Novick, R.P. (1995). Autocrine regulation of toxin synthesis by Staphylococcus aureus Proc. Nat. Acad. Sci. 92, 1619–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balaban, N.; Collins, L.V.; Cullor, J.S.; Hume, E.B.; Medina-Acosta, E.; Vieira da Motta, O.; O’Callaghan, R.; Rossitto, P.V.; Shirtliff, M.E.; Serafim da Silveira, L.; Tarkowski, A.; Torres, J.V. (2000). Prevention of diseases caused by Staphylococcus aureus using the peptide RIP. Peptides 21, 1301–11. [DOI] [PubMed] [Google Scholar]
- 5.Bergdoll, M.S. (1973). Enterotoxin detection. In: Hobbs, B.C. & Christian, J.H.B., ed. The microbiological safety of food. Academic Press, London, p. 287–92. [Google Scholar]
- 6.Braga, L.C.; Shupp, J.W.; Cummings, C.; Jett, M.; Takahashi, J.A.; Carmo, L.S.; Chartone-Souza, E.; Nascimento, A.M.A. (2004). Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production J. Ethnopharmacol. 96(1–2), 335–339. [DOI] [PubMed] [Google Scholar]
- 7.Cancelieri, N.M.; Vieira, I.J.C.; Schripsema, J.; Mathias, L.; Braz-Filho, R. (2002). Darcyribeirine, a novel pentacyclic indole alkaloid from Rauvolfia grandiflora Mart. Tetrahedron Lett. 43, 1783–1787. [Google Scholar]
- 8.Cancelieri, N.M.; Vieira, I.J.C.; Mathias, L.; Braz-Filho, R. (2003). 1H and 13C NMR structure determination of a new stereoisomer of isoreserpiline pseundoxyl fromRauvolfia grandiflora Magn. Reson. Chem., 41, 287–290. [Google Scholar]
- 9.Conegero, L.S.; Ide, R.M.; Nazari, A.S.; Sarragiotto, M.H.; Filho, B.P.D.; Nakamura, C.V. (2003). Constituintes químicos de Alchornea glandulosa (Euphorbiaceae). Quim. Nova 26, (6), 825–827. [Google Scholar]
- 10.Dempsey D.M.A.; Silva H.; Klessig D.F. (1998): Engineering Disease and Pest Resistance in Plants: Trends Microbiol 6, 54–61. [DOI] [PubMed] [Google Scholar]
- 11.Ferens, W.A.; Goff, W.L.; Davis, W.C.; Fox, L.K.; Deobald, C.; Hamilton, M.J.; Bohach, G.A. (1998). Induction of type 2 cytokines by a staphylococcal enterotoxin superantigen. J. Nat. Toxins 7, 193–213. [PubMed] [Google Scholar]
- 12.Furuya, E.Y.; Lowy, F.D. (2006). Antimicrobial-resistant bacteria in the community setting. Nat. Rev. Microbiol. 4, 36–45. [DOI] [PubMed] [Google Scholar]
- 13.Huber, B.; Eberl, L.; Feucht, W.; Polster, J. (2003). Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Z. Naturforsch [C] 58, 879–84. [DOI] [PubMed] [Google Scholar]
- 14.Kahl, B.C.; Goulian, M.; van Wamel, W.; Herrmann, M.; Simon, S.M.; Kaplan, G.; Peters, G.; Cheung, A.L. (2000). Staphylococcus aureus RN6390 replicates and induces apoptosis in a pulmonary epithelial cell line. Infec. Immun. 68, 5385– 5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karakawa, W.W.; Vann, W.F. (1982). Capsular polysaccharides of Staphylococcus aureus Sem. Infec. Dis. 4, 285–293. [Google Scholar]
- 16.Le Loir, Y.; Baron, F.; Gautier, M. (2003). Staphylococcus aureus and food poisoning. Gen. Mol. Res. 2, 63–76. [PubMed] [Google Scholar]
- 17.Lemos, G.C.S.; Oliveira, L.O.; Eberli, B.B.; Vieira-da-Motta, O; Folly, M.M. (2000). Bactericidal activity of macela (Achyrocline satureioides (Lam.) DC.) and jaborandi-falso (Piper aduncum L.) against strains of Staphylococcus aureus isolated from subclinical mastitis. Braz. J. Med. Plants 3, 67–72. [Google Scholar]
- 18.Lina, G.; Bohach, G.A.; Nair, S.P.; Hiramatsu, K. (2004). Standard nomenclature for the superantigens expressed by Staphylococcus J. Infec. Dis. 189, 2334–2336. [DOI] [PubMed] [Google Scholar]
- 19.Kiran, M.D.; Adikesavan, N.V.; Cirioni, O.; Giacometti, A.; Silvestri, C.; Scalise, G.; Ghiselli, R.; Saba, V.; Orlando, F.; Shoham, M.; Balaban, N. (2008). Discovery of a quorum-sensing inhibitor of drug-resistant staphylococcal infections by structure-based virtual screening. Mol. Pharmacol. 73:1578–1586. [DOI] [PubMed] [Google Scholar]
- 20.Monday, S.R.; Bohach, G.A. (1999). Properties of Staphylococcus aureus enterotoxins and toxic shock syndrome toxin-1. In: The comprehensive sourcebook of bacterial protein toxins. eds Alouf, J.E. and Freer, J.H. Academic Press, London, pp. 589–610. [Google Scholar]
- 21.National Committee for Clinical Laboratory Standards (2003). Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard. 8th Edition.
- 22.Nostro, A.; Cannatelli, M.A; Musolino, A.D.; Procopio, F.; Alonzo, V. (2002). Helichrysum italicum extract interferes with the production of enterotoxins by Staphylococcus aureus Lett. Appl. Microbiol. 35, 181–184. [DOI] [PubMed] [Google Scholar]
- 23.Novick, R.P. (2003). Auto induction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 48, 1429–1449. [DOI] [PubMed] [Google Scholar]
- 24.Olsen, S.J.; MacKinen, L.C.; Goulding, J.S.; Bean, N.H.; Stutsker, L. (2000). Surveillance for foodborne disease outbreaks – United States, 1993–1997. Morb. Mort. Wkly. Rep. 49, (SS01), 1–51. [PubMed] [Google Scholar]
- 25.Omoe, K.; Hu, D.-L.; Takahashi-Omoe, H.; Nakane, A.; Shinagawa, K. (2005). Comprehensive analysis of classical and newly described staphylococcal superantigenic toxin genes in Staphylococcus aureus isolates. FEMS Microbiol. Lett. 246, 191–198. [DOI] [PubMed] [Google Scholar]
- 26.Peterson, P.K.; Wilkinson, B.J.; Kim, Y.; Schmeling, D.; Quie, P.G. (1978). Influence of encapsulation on staphylococcal opsonization and phagocytosis by human polymorphonuclear leukocytes. Infect. Immun. 19, 943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poutrel, B.; Boutonnier, A.; Sutra, L.; Fournier, J.M. (1988). Prevalence of capsular polysaccharide types 5 and 8 among Staphylococcus aureus isolates from cow, goat, and ewe milk. J. Clin. Microbiol. 26, 38–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tagana, J.C; Rivera, W.L. (2008). Epigallocatechin gallate from Camellia sinensis L. (Kuntze) is a potential quorum sensing inhibitor in Chromobacterium violaceum Sci. Diliman 20, 24–30. [Google Scholar]
- 29.Tanaka, J.C.A.; da Silva, C.C.; de Oliveira, A.J.B.; Nakamura, C.V.; Dias Filho, B.P. (2006). Antibacterial activity of indole alkaloids from Aspidosperma ramiflorum. Braz. J. Med. Biol. Res. 39, 387–391. [DOI] [PubMed] [Google Scholar]
- 30.Thakker, M., Park, J.S.; Carey, V.; Lee, J.C. (1998). Staphylococcus aureus serotype 5 capsular polysaccharide is anti-phagocytic and enhances bacterial virulence in a murine bacteremia model. Infect. Immun. 66, 5183–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas, D.Y.; Jarraud, S.; Lemercier, B.; Cozon, G.; Echasserieau, K.; Etienne, J.; Gougeon, M.L.; Lina, G.; Vandenesch, F. (2006). Staphylococcal enterotoxin-like toxins U2 and V, two new staphylococcal superantigens arising from recombination within the enterotoxin gene cluster. Infect. Immun. 74, 4724– 4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todhunter, D.A.; Cantwell, L.L.; Smith, K.L.; Hoblet, K.H.; Hogan, J.S. (1993). Characteristics of coagulase-negative Staphylococci isolated from bovine intramammary infections. Vet. Microbiol. 34, 373–380. [DOI] [PubMed] [Google Scholar]
- 33.Uhl, M.V.C.; Bottecchia, R.J.; Azevedo-Silva, J.; Antonio, D.L.; Vieira-da-Motta, O.; Mittmann, J.; Damasceno Ribeiro, P.; De Souza Campos Fernandes, R.C.; Távora, N.; Medina-Acosta, E. (2004). Suitability of a recombinant Staphylococcus aureus enterotoxin C bovine variant for immunodiagnostics and therapeutic vaccine development. Vaccine 22, 4191–4202. [DOI] [PubMed] [Google Scholar]
- 34.Vieira-da-Motta, O.; Folly, M.M.; Sakyiama, C.C.H. (2001a). Detection of different Staphylococcus aureus strains in bovine milk from subclinical mastitis using PCR and routine techniques. Braz. J. Microbiol. 32(1), 27–31. [Google Scholar]
- 35.Vieira-da-Motta, O.; Damasceno, P.R.; Dias da Silva, W.; Medina-Acosta, E. (2001b) RNAIII Inhibiting Peptide (RIP) inhibits agr-regulated toxin production. Peptides 22, 1621–1627. [DOI] [PubMed] [Google Scholar]
- 36.Vieira-da-Motta, O.; Medina-Acosta, E.; Almeida, C.M.C.; Kipnis, T.L.; Dias da Silva, W. (2001c). Development of anti-Staphylococcus aureus enterotoxins antibodies in chickens and their purification from yolk. Scand. J. Immun. 54, Suppl 1, 117. [Google Scholar]
- 37.von Eiff, C.; Peters, G.; Heilmann, C. (2002). Pathogenesis of infections due to coagulase-negative staphylococci. The Lancet Infect. Dis. 2, 677–685. [DOI] [PubMed] [Google Scholar]
- 38.Vuong, C.; Götz, F.; Otto, M. (2000). Construction and characterization of an agr deletion mutant of Staphylococcus epidermidis Infect. Immun. 68, 1048–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarwood, J.M.; Schlievert, P.M. (2003). Quorum sensing in Staphylococcus infections. J. Clin. Invest. 112, 1620–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zecconi, A.; Cesaris, L.; Liandris, E.; Daprà, V.; Piccinini, R. (2006). Role of several Staphylococcus aureus virulence factors on the inflammatory response in bovine mammary gland. Microb. Pathogen 40, 177–183. [DOI] [PubMed] [Google Scholar]


