Abstract
The work herewith investigated the production of yeast biomass as a source of protein, using Yarrowia lipolytica NRRL YB-423 and raw glycerol from biodiesel synthesis as the main carbon source. A significant influence of glycerol concentration, initial pH and yeast extract concentration on biomass and protein content was observed according to the 2v5-1 fractional design. These factors were further evaluated using a central composite design and response surface methodology, and an empirical model for protein content was established and validated. The biomass of Yarrowia lipolytica NRRL YB-423 reached 19.5 ± 1.0 g/L in shaken flasks cultivation, with a protein content of 20.1 ± 0.6% (w/w).
Keywords: Raw glycerol, single cell protein, yeast biomass, Yarrowia lipolytica
INTRODUCTION
Within the products that can be a substitute for the protein supplement, the microorganisms (algae, bacteria, molds and yeasts) are considered a source of cell protein with an elevated protein content besides possessing a rapid growth rate and the possibility of being cultured on diverse substrates (4).
Single cell protein (SCP) is the manufacture of cell mass using microorganisms grown in large scale culture systems. After cultivation biomass is harvested and may be subjected to downstream processing steps like washing, cell disruption, protein extraction and purification. It can be used for protein supplementation of a staple diet by replacing costly conventional sources like soymeal and fishmeal to alleviate the problem of protein scarcity (2). This activity represents a promising application of biotechnology, which is even more successful when associated to the utilization of sewage or industrial wastes as substrate (14).
The production of yeast biomass is advantageous because of its nontoxic nature and its high productivity. Yeasts provide the B-complex group of vitamins and they also show a low level of nucleic acid content (20). Among yeasts, Saccharomyces and Candida are classified among the most interesting microorganisms for their protein content (23). However, other yeasts, for example Yarrowia genus, show a lower but useful protein content, with a content of essential amino acids complied with the FAO standards (9). In addition, this biomass also can be a source of essential fatty acids (12). On the other hand, an important aspect in the production of yeast biomass as a source of nutrients is the development of a culture medium based on low cost substrates with high yield and productivity.
A variety of substrates have been utilized to cultivate yeasts to obtain SCP, mostly cheap substrates, such as potato chips manufacturing (7), sugar cane hemicellulosic hydrolizate (13), rice polishings (15), glutamate fermentation wastewater (22) and orange peel extracts (23).
Biodiesel is produced by the transesterification of triglycerides with a monovalent alcohol, such as methanol and ethanol, to fatty acid alkyl esters, and glycerol is an inherent side product of this reaction. It is possible to calculate that, stoichiometrically, 10% (w/w) of glycerol is formed by this reaction. However, that value is for pure glycerol. The raw glycerol that falls free from biodiesel synthesis usually presents 55-90% of purity. The rest of the raw glycerol consists of unconverted triglycerides, unconverted methanol or ethanol, biodiesel, soaps and contamination. Therefore, this crude glycerol contains too many contaminants for a useful application in chemistry or pharmacy and a purification treatment is needed (1).
The current annual amount of glycerol arising from the biodiesel production exceeds the world market for pure glycerol with high quality for industrial applications (chemical and pharmaceutical). As a consequence, prices have fallen and many companies worldwide that chemically produced glycerol have shut down business. Some biodiesel companies have severe problems getting rid the excess glycerol and disposal is quite expensive. This way, glycerol is becoming an important feedstock and an abundant renewable carbon source for microbial cultivation (1, 19).
Therefore, glycerol from biodiesel production would be a good alternative to be used as a competitive substrate for biomass production since it is a byproduct and consequently its price is much lower than traditional carbon sources, such as glucose, sucrose and starch. Moreover, glycerol bioconversion adds significant value to the productive chain of the biodiesel industry, contributing to their competitiveness (19). However, although utilization of raw glycerol in the culture medium without prior purification offers a remarkable advantage against the use of pure glycerol as substrate, only few reports have appeared in the literature on the use of this substrate as carbon source (12).
In the present work, a 2v5-1 fractional design followed by a central composite design has been used to establish the medium composition for the yeast Yarrowia lipolytica NRRL YB-423 growing on a raw glycerol-based medium, in order to maximize biomass concentration and protein content.
MATERIALS AND METHODS
Microorganism
Yarrowia lipolytica NRRL YB-423 was provided by the Northern Regional Research Laboratory (Peoria, USA) and certified as GRAS (Generally Recognized As Safe). This strain was previously selected among other strains according to their growth capabilities on glycerol (17). The yeast was maintained on Yeast Malt (YM) Agar and stored at 4°C.
Raw Glycerol
Raw glycerol was obtained from the synthesis of biodiesel by transesterification of soybean oil and anhydrous ethanol in alkaline catalysis. The transesterification reaction was performed using an ethanol/soybean oil molar ratio of 6:1 and 0.1% w/v of sodium hydroxide as an alkaline catalyst. The reaction was carried out at 60°C for 120 min. Conventional procedures were used for glycerol separation, such as neutralization with sulfuric acid, filtration, decantation and evaporation of residual ethanol (18). The raw glycerol contained 79% (w/w) of glycerol. The amount of raw glycerol to be added considered its composition in order to result the required substrate concentration.
Inoculum
Two tubes of microbial culture, previously incubated at 25°C for 48 h, were used. They were scraped with 10 mL of 0.1% (w/v) peptone diluent for each tube and transferred to 500 mL Erlenmeyer flask containing 200 mL of medium proposed by Papanikolaou and Aggelis (10), containing (g/L): 30 pure glycerol; 7 KH2PO4; 2.5 Na2HPO4; 1.5 MgSO4.7H2O; 0.15 CaCl2; 0.15 FeCl3.6H2O; 0.02 ZnSO4.7H2O; 0.06 MnSO4.H2O; 0.5 (NH4)2SO4; 0.5 yeast extract; pH adjusted to 6.0. The suspension was incubated at 30°C and 180 rpm, and growth was monitored by counting in a Neubauer chamber (21).
Shaken Flasks Cultivation
The flasks containing raw glycerol-based medium were inoculated with yeast suspension previously prepared, in order to achieve 1x107 cells/mL. The flasks were maintained in a rotary shaker at 30°C and 180 rpm. Samples were taken at regular intervals and analytical determinations were performed in duplicate. Maximum biomass concentration (g/L) and protein content (% w/w) at the end of cultivation were obtained as responses.
Experimental Design
A 2v5-1 fractional design was carried out in order to evaluate the effects of the variables of the culture medium, based on the medium proposed by Rivaldi et al. (16). The variables were: concentrations of glycerol (30 to 60 g/L), diammonium hydrogen phosphate (5.5 to 12.5 g/L), yeast extract (0.5 to 1.5 g/L) and peptone (0.5 to 1.5 g/L) and initial pH (4.5 to 6.5). The concentrations of potassium dihydrogen phosphate (5.5 g/L), ammonium sulfate (1.0 g/L), magnesium sulfate (0.25 g/L) and calcium chloride dihydrate (0.021 g/L) were maintained.
With the most influential variables, a 23 central composite design was proposed, in order to enhance biomass production and protein content. The variables analyzed were concentrations of glycerol (from 16.1 to 33.9 g/L) and yeast extract (from 0.6 to 2.4 g/L) and initial pH of the medium (from 4.8 to 7.2). The quantities of diammonium hydrogen phosphate and peptone were fixed at 5.5 g/L and 1.5 g/L, respectively.
All the experiments were carried out in a randomized way. In the best conditions, the cultivations were performed in triplicate in order to validate the mathematical model for protein content at 72 h of cultivation.
Data Analysis
Statistica 6.0 software (StatSoft Inc., USA) was used for the experimental designs and statistical analysis of the experimental data. The analysis of variance (ANOVA) was used to estimate the statistical parameters.
Biomass Concentration
The biomass was monitored by measuring the absorbance at 600 nm. Samples were centrifuged at 1780*g for 15 min and cells were recovered after washing twice with distilled water. A calibration curve between OD600 and the cell dry-weight concentration (g/L) was first established.
Protein Content
Protein content was determined by the Kjeldahl method, using a factor of 6.25 to convert nitrogen to protein content (3).
pH
The pH of the supernatant was measured using a pH meter, according to AOAC (3).
RESULTS AND DISCUSSION
Fractional Design
Table 1 presents the assays of 2v5-1 fractional design with coded and real values of each variable and with the responses protein content and maximum biomass concentration. Trial 3 showed higher biomass production during cultivation, which produced 21.8 g/L of biomass with protein content of 9.6% (w/w), using 30 g/L of glycerol, 5.5 g/L of diammonium hydrogen phosphate, 0.5 g/L of yeast extract, 0.5 g/L of peptone and initial pH of 6.5. The best protein content was 18.4% (w/w) in trial 13, producing 12 g/L of biomass in a medium containing 30 g/L of glycerol, 12.5 g/L of diammonium hydrogen phosphate, 1.5 g/L of yeast extract, 1.5 g/L of peptone and initial pH of 4.5. However, trial 1 (glycerol 30 g/L, diammonium hydrogen phosphate 5.5 g/L, yeast extract 0.5 g/L, peptone 1.5 g/L and initial pH 4.5) reached high levels for both, protein content (18.2% w/w) and biomass concentration (17.3 g/L).
Table 1.
Coded values, real values (in parentheses) and experimental data obtained from the assays in the 2v5-1 fractional design.
| Trial | Glycerol (g/L) | pH | DAP* (g/L) | YE** (g/L) | Peptone (g/L) | Biomass concentration (g/L) | Protein content (% w/w) |
|---|---|---|---|---|---|---|---|
| 1 | -1 (30) | -1 (4.5) | -1 (5.5) | -1 (0.5) | +1 (1.5) | 17.3 | 18.2 |
| 2 | +1 (60) | -1 (4.5) | -1 (5.5) | -1 (0.5) | -1 (0.5) | 4.5 | 13.5 |
| 3 | -1 (30) | +1 (6.5) | -1 (5.5) | -1 (0.5) | -1 (0.5) | 21.8 | 9.6 |
| 4 | +1 (60) | +1 (6.5) | -1 (5.5) | -1 (0.5) | +1 (1.5) | 18.7 | 8.4 |
| 5 | -1 (30) | -1 (4.5) | +1 (12.5) | -1 (0.5) | -1 (0.5) | 13.9 | 17.0 |
| 6 | +1 (60) | -1 (4.5) | +1 (12.5) | -1 (0.5) | +1 (1.5) | 7.3 | 8.4 |
| 7 | -1 (30) | +1 (6.5) | +1 (12.5) | -1 (0.5) | +1 (1.5) | 18.8 | 13.6 |
| 8 | +1 (60) | +1 (6.5) | +1 (12.5) | -1 (0.5) | -1 (0.5) | 15.4 | 7.9 |
| 9 | -1 (30) | -1 (4.5) | -1 (5.5) | +1 (1.5) | -1 (0.5) | 14.5 | 16.0 |
| 10 | +1 (60) | -1 (4.5) | -1 (5.5) | +1 (1.5) | +1 (1.5) | 7.8 | 12.2 |
| 11 | -1 (30) | +1 (6.5) | -1 (5.5) | +1 (1.5) | +1 (1.5) | 15.1 | 16.7 |
| 12 | +1 (60) | +1 (6.5) | -1 (5.5) | +1 (1.5) | -1 (0.5) | 10.3 | 9.8 |
| 13 | -1 (30) | -1 (4.5) | +1 (12.5) | +1 (1.5) | +1 (1.5) | 12.0 | 18.4 |
| 14 | +1 (60) | -1 (4.5) | +1 (12.5) | +1 (1.5) | -1 (0.5) | 2.7 | 13.3 |
| 15 | -1 (30) | +1 (6.5) | +1 (12.5) | +1 (1.5) | -1 (0.5) | 11.6 | 15.1 |
| 16 | +1 (60) | +1 (6.5) | +1 (12.5) | +1 (1.5) | +1 (1.5) | 11.8 | 10.0 |
| 17 | 0 (45) | 0 (5.5) | 0 (9) | 0 (1) | 0 (1) | 14.3 | 16.0 |
| 18 | 0 (45) | 0 (5.5) | 0 (9) | 0 (1) | 0 (1) | 14.5 | 16.3 |
| 19 | 0 (45) | 0 (5.5) | 0 (9) | 0 (1) | 0 (1) | 14.3 | 15.1 |
DAP: diammonium hydrogen phosphate
YE: yeast extract
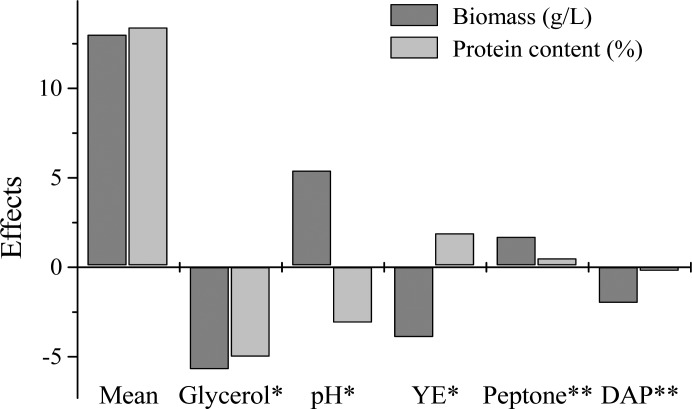
Figure 1 shows the effects of variables on the maximum biomass concentration and protein content for Yarrowia lipolytica NRRL YB-423. It was observed that all variables had significant effect on biomass concentration, at the 95% confidence level (p<0.05). However, the increase from 30 to 60 g/L of glycerol, from 5.5 to 12.5 g/L of diammonium hydrogen phosphate and from 0.5 to 1.5 g/L of yeast extract led to a decrease of 5.5 g/L, 1.8 g/L and 3.8 g/L in biomass concentration, respectively. The variables initial pH and peptone concentration showed opposite effects. With the change from the level -1 to the level +1, biomass concentration increased 5.1 g/L and 2.0 g/L, respectively.
Figure 1.
Estimates of the effects of the variables of the culture medium. *Statistically significant effect (p<0.05) for both biomass concentration and protein content; **Statistically significant effect (p<0.05) for biomass concentration.
In relation to the protein content, the variable glycerol concentration had a significant decreasing effect (p<0.05), decreasing 5.1%. The increases of initial pH and yeast extract were significant (p<0.05). With the change from level -1 to +1 in pH and yeast extract, protein content decreased 3.2% and increased 1.9%, respectively.
This way the chosen variables for the central composite design that most influenced the maximum biomass concentration and protein content included: glycerol concentration, initial pH of the medium and yeast extract concentration.
The variables diammonium hydrogen phosphate and peptone were not studied in the central composite design, since they had no significant effects on protein and resulted in lower impact on the maximum biomass concentration. Thus, diammonium hydrogen phosphate and peptone were fixed at 5.5 g/L and 1.5 g/L (levels -1 and +1, respectively) because these levels favored biomass production.
Central Composite Design
A 23 central composite design, a total of 11 trials (three replicates at the central point), was performed with the variables: initial pH, concentrations of glycerol and yeast extract. The assays are presented in Table 2. It can be observed that the greater protein content (21.9%) was obtained in trial 3, corresponding to 16.1 g/L of glycerol, 0.6 g/L of yeast extract and initial pH of 7.2. In the condition of trial 2 (glycerol 33.9 g/L, yeast extract 0.6 g/L and initial pH 4.8) the highest value for maximum biomass (23.2 g/L) was reached.
Table 2.
Central composite design with coded values and real values (in parenthesis).
| Trial | Glycerol (g/L) | pH | YE* (g/L) | Biomass concentration (g/L) | Protein content (% w/w) | Predicted protein content (% w/w) | Relative deviation (%) |
|---|---|---|---|---|---|---|---|
| 1 | -1 (16.1) | -1 (4.8) | -1 (0.6) | 17.0 | 18.4 | 18.4 | 0.0 |
| 2 | +1 (33.9) | -1 (4.8) | -1 (0.6) | 23.2 | 20.2 | 19.4 | 4.0 |
| 3 | -1 (16.1) | +1 (7.2) | -1 (0.6) | 20.2 | 21.9 | 21.2 | 3.2 |
| 4 | +1 (33.9) | +1 (7.2) | -1 (0.6) | 22.5 | 20.4 | 20.2 | 1.0 |
| 5 | -1 (16.1) | -1 (4.8) | +1 (2.4) | 21.0 | 16.0 | 15.2 | 5.0 |
| 6 | +1 (33.9) | -1 (4.8) | +1 (2.4) | 19.7 | 16.0 | 16.2 | -1.3 |
| 7 | -1 (16.1) | +1 (7.2) | +1 (2.4) | 14.6 | 20.2 | 19.6 | 3.0 |
| 8 | +1 (33.9) | +1 (7.2) | +1 (2.4) | 22.7 | 18.7 | 18.6 | 0.5 |
| 9 | 0 (25) | 0 (6) | 0 (1.5) | 15.7 | 17.2 | 18.6 | -8.1 |
| 10 | 0 (25) | 0 (6) | 0 (1.5) | 16.8 | 17.5 | 18.6 | -6.3 |
| 11 | 0 (25) | 0 (6) | 0 (1.5) | 15.7 | 17.5 | 18.6 | -6.3 |
YE: yeast extract
The values obtained for maximum biomass concentration of Yarrowia lipolytica NRRL YB-423 growing on raw glycerol-based medium were significant when compared to those cited in literature. Papanikolaou et al. (12) reached 7.1 g/L at 92 h of cultivation using Yarrowia lipolytica ACA-DC 50,109 growing on a medium containing raw glycerol (20.5 g/L) from biodiesel synthesis. Musial et al. (9) observed a maximum biomass concentration of 19 g/L for Yarrowia lipolytica A-101 but using a medium containing 20 g/L of crude rapeseed oil as the main carbon source.
On the other hand, the protein content was lower compared with other microorganisms used for single cell protein production. Choi et al. (5) observed a protein content of 31% (w/w) using Kluyveromyces marxianus KCTC 7118 growing on YM broth with glucose. A protein content of 32.75% (w/w) was obtained when Candida utilis PP Y 12 was cultivated on defatted rice polishings (15).
However, the present results represent an important increase compared with our previous work (17). Using pure glycerol and the medium proposed by Papanikolaou and Aggelis (10) we reached maximum biomass concentration of 13.8 g/L and protein content of 13.6% (w/w).
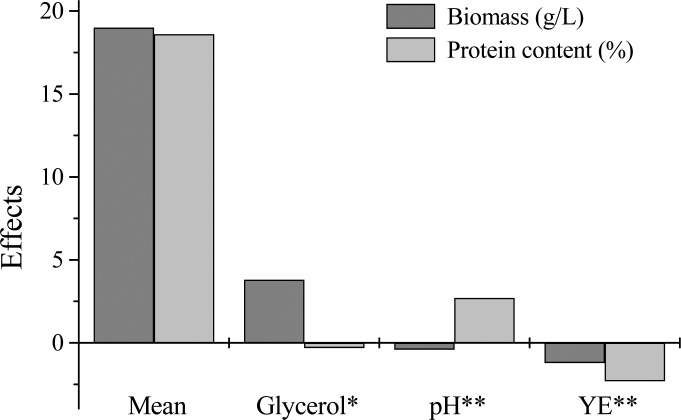
Figure 2 presents the effects of the variables of the culture medium on the biomass concentration and protein content. It can be seen that only glycerol concentration had a significant effect on biomass concentration among the variables, at 95% confidence level. An increase from 16.1 to 33.9 g/L of glycerol increased biomass in 3.8 g/L. In relation to the protein content, the variables initial pH and yeast extract concentration had significant effects (p<0.1). With the change from level -1 to +1 in pH and yeast extract, protein content increased 2.7% and decreased 2.3%, respectively.
Based on the analysis of variance (ANOVA), as shown in Table 3, a predictive model could not be established for biomass concentration, since its calculated F value was less than the critical F value and the regression coefficient was low (0.54).
Table 3.
ANOVA for the central composite design.
| Source of variation | Sums of squares | Degrees of freedom | Mean squares | F-test | ||||
|---|---|---|---|---|---|---|---|---|
| Biomass | Protein content | Biomass | Protein content | Biomass | Protein content | Biomass | Protein content | |
| Regression | 29.26 | 29.19 | 1 | 4 | 29.26 | 7.30 | 3.75a | 6.38a |
| Residual | 70.23 | 6.86 | 9 | 6 | 7.80 | 1.14 | ||
| Lack of fit | 69.42 | 6.80 | 7 | 4 | 9.92 | 1.70 | 24.59b | 56.64b |
| Pure error | 0.81 | 0.06 | 2 | 2 | 0.40 | 0.03 | ||
| Total | 99.49 | 36.04 | 10 | 10 | ||||
Protein content: r = 0.90; F0.9;4;6 = 3.18; F0.9;4;2 = 9.24.
Biomass concentration: r = 0.54; F0.95;1;9 = 5.12; F0.95;7;2 = 19.35.
F-ratio (regression/residual).
F-ratio (lack of fit/pure error).
However, according to ANOVA (Table 3), a first order model was established for protein content. The correlation coefficient was 0.90 and the F-value was 2 times higher than the listed value for the 90% confidence level. A polynomial equation was generated (Equation 1) in order to describe the protein content at 72 h of cultivation as a function of the studied variables.
Protein content (% w/w) = 18.6 + 1.3(pH) - 1.2(YE) - 0.5(glycerol x pH) + 0.4 (pH x YE) (1)
This model predicted values for protein content very well, with relative deviations lower than 10% in relation to those obtained experimentally for all conditions (Table 2).
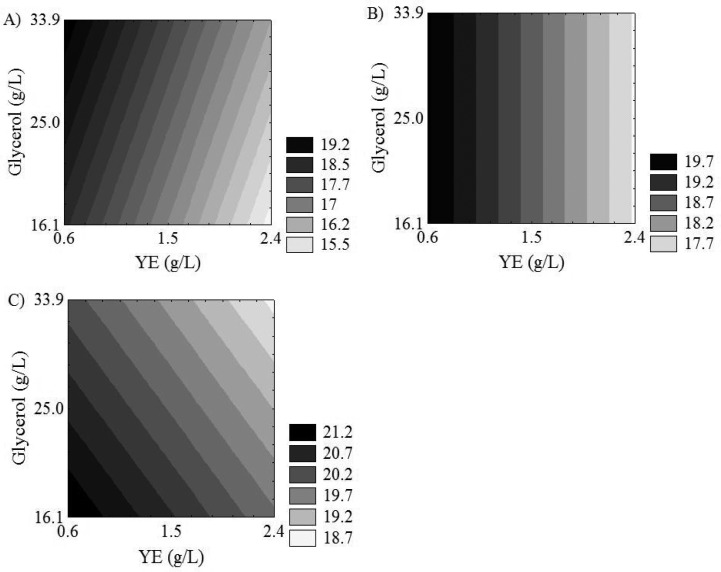
Figure 3 presented contour plots for protein content. According to Figures 3A, 3B and 3C, a low yeast extract concentration (0.6 g/L) favored an increase in protein content.
Figure 3.
Contour plots for protein content as a function of glycerol and yeast extract (YE) concentrations. Initial pH equal to 4.8 (A); 6.0 (B); and 7.2 (C).
When the initial pH was low (4.8), the increase in glycerol concentration favored the protein content (Figure 3A), reaching 19% (w/w). An opposite behavior was observed in Figure 3C. When pH was high (7.2), glycerol in low concentration (16.1 g/L) increased the protein content, reaching values higher than 21% (w/w). On the order hand, when pH was 6.0 (intermediate level), glycerol concentration did not influence the protein content (Figure 3B).
So, according to Figure 3, it was verified that a decrease in yeast extract concentration (0.6 g/L) increases the protein content, but glycerol concentration and initial pH influenced protein content in different ways.
Thus, to validate the model for protein content, trial 2 was chosen. This assay corresponds to a higher glycerol concentration (33.9 g/L) that favored biomass production (according to Figure 2), combined with a lower pH value (4.8) that favored protein content, maintaining a lower yeast extract concentration (0.6 g/L) (Figure 3A).
Figure 2.
Estimates of the effects of glycerol concentration, initial pH and yeast extract (YE) concentration. *Statistically significant effect (p<0.05) for biomass concentration; **Statistically significant effect (p<0.1) for protein content.
Validation of the Empirical Model for Protein Content
In order to confirm the validity of the model, three additional experiments in shaken flasks were performed using the culture conditions of trial 2. Maximum biomass concentration was reached at 54 h of cultivation (19.5 ± 1.0 g/L). During cultivation, a decrease in pH was clearly observed (data not shown), probably associated with the production of organic acids such as pyruvic acid (6, 8), citric and isocitric acids (11).
The experimental results for protein content were 20.5, 20.3 and 19.4% (w/w), while the model predicted 19.4% (w/w). So, the relative deviations were 5.4, 4.4 and 0.0%, respectively, showing that the predicted value for protein content agreed well with the experimental values. This fact demonstrates the validity of the empirical model for protein content.
CONCLUSION
A 2v5-1 fractional design followed by a central composite design has been proved to be effective in establishing the medium composition for the yeast Yarrowia lipolytica NRRL YB-423 using raw glycerol as the main carbon source. The final composition of the culture medium was as follows: initial pH 4.8, glycerol 33.9 g/L, yeast extract 0.6 g/L, diammonium hydrogen phosphate 5.5 g/L, peptone 1.5 g/L, potassium dihydrogen phosphate 5.5 g/L, ammonium sulfate 1g/L, magnesium sulfate 0.25 g/L, calcium chloride dihydrate 0.021 g/L. In this culture condition, a biomass concentration of 19.5 ± 1.0 g/L and a protein content of 20.1 ± 0.6% (w/w) were reached. These results represent a 1.4-fold increase in biomass concentration and 1.5-fold increase in protein content in relation to our previous work. Thus, Yarrowia lipolytica NRRL YB-423 may have highly potential application for industrial production of yeast biomass using raw glycerol.
Acknowledgments
The authors would like to thank FAPERGS (Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for their financial support.
REFERENCES
- 1.Amaral P.F.F., Ferreira T.F., Fontes G.C., Coelho M.A.Z. Glycerol valorization: new biotechnological routes. Food Bioprod. Process. 2009;87(3):179–186. [Google Scholar]
- 2.Anupama, Ravindra P. Value-added food: single cell protein. Biotechnol. Adv. 2000;18(6):459–479. doi: 10.1016/s0734-9750(00)00045-8. [DOI] [PubMed] [Google Scholar]
- 3.AOAC. Official Methods of Analysis. 17. Washington, D.C.: Association of Official Analytical Chemists; 2000. [Google Scholar]
- 4.Araújo L.F., Medeiros A.N., Neto A.P., Oliveira L.S.C., Silva F.L.H. Protein enrichment of cactus pear (Opuntia ficus - indica Mill) using Saccharomyces cerevisiae in solid-state fermentation. Braz. Arch. Biol. Techn. 2005;48:161–168. [Google Scholar]
- 5.Choi M.H, Ji G.E., Koh K.H., Ryu Y.W., Jo D.H., Park Y.H. Use of waste Chinese cabbage as a substrate for yeast biomass production. Bioresource Technol. 2002;83(3):251–253. doi: 10.1016/s0960-8524(01)00232-2. [DOI] [PubMed] [Google Scholar]
- 6.Finogenova T.V., Morgunov I.G., Kamzolova S.V., Chernyavskaya O.G. Organic acid production by the yeast Yarrowia lipolytica: a review of prospects. Appl. Biochem. Microbiol. 2005;41(5):418–425. [PubMed] [Google Scholar]
- 7.Gélinas P., Barrete J. Protein enrichment of potato processing waste through yeast fermentation. Bioresource Technol. 2007;98(5):1138–1143. doi: 10.1016/j.biortech.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Morgunov I.G., Kamzolova S.V., Perevoznikova O.A., Shishkanova N.V., Finogenova T.V. Pyruvic acid production by a thiamine auxotroph of Yarrowia lipolytica. Proc. Biochem. 2004;39(11):1469–1474. [Google Scholar]
- 9.Musial I., Rymowicz W., Cibis E. Optimization of single-cell-biomass production by Yarrowia lipolytica using response surface methodology and pulse method. EJPAU. 2004 [Google Scholar]
- 10.Papanikolaou S., Aggelis G. Lipid production by Yarrowia lipolytica growing on industrial glycerol in a single-stage continuous culture. Bioresource Technol. 2002;82(1):43–49. doi: 10.1016/s0960-8524(01)00149-3. [DOI] [PubMed] [Google Scholar]
- 11.Papanikolaou S., Muniglia L., Chevalot I., Aggelis G., Marc I. Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J. Appl. Microbiol. 2002;92(4):737–744. doi: 10.1046/j.1365-2672.2002.01577.x. [DOI] [PubMed] [Google Scholar]
- 12.Papanikolaou S., Fakas S., Fick M., Chevalot I., Galiotou-Panayotou M., Komaitis M., Marc I., Aggelis G. Biotechnological valorization of raw glycerol discharged after bio-diesel (fatty acid methyl esters) manufacturing process: production of 1,3-propanediol, citric acid and single cell oil. Biomass Bioenerg. 2008;32(1):60–71. [Google Scholar]
- 13.Pessoa Jr. A., Mancilha I.M., Sato S. Cultivation of Candida tropicalis in sugar cane hemicellulosic hydrolyzate for microbial protein production. J. Biotechnol. 1996;51(1):83–88. [Google Scholar]
- 14.Ponsano E.H.G., Lacava P.M., Pinto M.F. Chemical composition of Rhodocyclus gelatinosus biomass produced in poultry slaughterhouse wastewater. Braz. Arch. Biol. Techn. 2003;46(2):143–147. [Google Scholar]
- 15.Rajoka M.I., Khan S.H., Jabbar M.A., Awan M.S., Hashmi A.S. Kinetics of batch single cell protein production from rice polishings with Candida utilis in continuously aerated tank reactors. Bioresource Technol. 2006;97(15):1934–1941. doi: 10.1016/j.biortech.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Rivaldi I.D., Fonseca R.R., Silva S.S. Utilização do subproduto da produção de biodiesel para obtenção de biomassa e biomoléculas. XVI Simpósio Nacional de Bioprocessos; Curitiba, PR, Brazil. 2007. [Google Scholar]
- 17.Santos E.O., Michelon M., Gallas J.A., Burkert J.F.M., Burkert C.A.V. Seleção de linhagens de leveduras para produção de biomassa usando glicerol como fonte de carbono. III Congresso de Ciências Farmacêuticas/III Simpósio em Ciência e Tecnologia de Alimentos do Mercosul; Cascavel, PR, Brazil. 2008. [Google Scholar]
- 18.Santos E.O., Rosa C.F.C., Mendonça A.P., D'Oca M.G.M., Villarreyes J.A.M. Produção batch de biodiesel por catálise alcalina II: proposta de um método de produção e purificação.. II Congresso Brasileiro de Plantas Oleaginosas, Óleos, Gorduras e Biodiesel; Varginha, MG, Brazil. [Google Scholar]
- 19.Silva G.P., Mack M., Contiero J. Glycerol: a promissing and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009;27(1):30–39. doi: 10.1016/j.biotechadv.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Uysal H., Aydogan M.N., Algur O.F. Effect of single cell protein as a protein source in Drosophila culture. Braz. J. Microbiol. 2002;33(4):314–317. [Google Scholar]
- 21.Zhang Y., Rittmann B.E., Wang J., Sheng Y., Yu J., Shi H., Qian Y. High-carbohydrate wastewater treatment by IAL-CHS with immobilized Candida tropicalis. Process Biochem. 2005;40(2):857–863. [Google Scholar]
- 22.Zheng S., Yang M., Yang Z., Yang Q. Biomass production from glutamate fermentation wastewater by the co-culture of Candida halophyla and Rhodotorula glutinis. Bioresource Technol. 2005;96(13):1522–1524. doi: 10.1016/j.biortech.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Ziino M., Curto R.B.L., Salvo F., Signorino D., Chiofalo B., Giuffrida D. Lipid composition of Geotrichum candidum single cell protein grown in continuous submerged culture. Bioresource Technol. 1999;67(1):7–11. [Google Scholar]