Abstract
Staphylococcus epidermidis is the most frequent cause of nosocomial sepsis and catheter-related infections, in which biofilm formation is considered to be the main virulence mechanism. In biofilm environment, microbes exhibit enhanced resistance to antimicrobial agents. This fact boosted the search of possible alternatives to antibiotics. Farnesol and N-acetylcysteine (NAC) are non-antibiotic drugs that have demonstrated antibacterial properties. In this study, the effect of farnesol and NAC isolated or in combination (farnesol+NAC) was evaluated. NAC at 10 × MIC caused a total cell death in planktonic cells. On the other hand, S. epidermidis biofilms exhibited 4 log reduction in viable cell number after a 24h treatment with NAC at the former concentration. Our results demonstrated that there was a higher CFU log reduction of S. epidermidis planktonic cells when farnesol was combined with NAC at 1 × MIC relatively to each agent alone. However, these results were not relevant because NAC alone at 10 × MIC was always the condition which gave the best results, having a very high killing effect on planktonic cells and a significant bactericidal effect on biofilm cells. This study demonstrated that no synergy was observed between farnesol and NAC. However, the pronounced antibacterial effect of NAC against S. epidermidis, on both lifestyles, indicates the use of NAC as a potential therapeutic agent in alternative to antibiotics.
Keywords: Nosocomial infection, biofilm, Staphylococci, farnesol, N- acetylcysteine.
INTRODUCTION
Staphylococcus epidermidis lives naturally on the skin and mucous membrane as a commensal of the human skin flora (9) and was primarely considered a natural human inhabitant bacterium with a low pathogenic potential (22). However, in recent decades, this bacterium was identified as a common cause of numerous infections on indwelling medical devices (22) and actually S. epidermidis is among the most leading causes of nosocomial infections (16). These bacteria form biofilms on implanted medical devices such as central venous catheters (CVCs), urinary catheters, prosthetic heart valves, orthopedic devices, contact lenses, etc, and cause persistent infections (21) and diseases such as septicemia and endocarditis (3). The biofilm-forming ability of Staphylococcus epidermidis has been considered to be its main virulence mechanism (5, 20) by which this organism is able to persist in infections/diseases (11). Many implant infections sometimes requires the implant removal, causing considerable suffering for the patient, with pain and disability and even increased mortality (8, 9). Moreover, the long-term systemic antibiotics, surgical debridement, and prolonged hospitalization, greatly increase the costs associated with implant replacement surgery.
The biofilm formation ability is a major clinical problem, mainly due to the intrinsic tolerance/resistance of biofilm cells to antibiotics (5). Antibiotic combination represents a therapeutic option in the treatment of S. epidermidis infections (14). However, increasing multiple resistance to antibiotics has made the development of new treatment options for serious infections a matter of urgent concern. In recent years, much research has been devoted to investigating possible alternatives to antibiotics, studying their mode of action and synergistic effects with other antimicrobial compounds. Farnesol is a sesquiterpene alcohol that has demonstrated to inhibit the growth of some microorganisms, such as Staphylococcus aureus and Streptococcus mutans, evidencing its potential use as antimicrobial agent (6, 11). The mechanism of action of this sesquiterpenoid probably involves cell membrane damages (6, 11, 13). N-acetylcysteine is another non-antibiotic drug that has antibacterial properties (17). NAC is one of the smallest drug molecules in use and it is generally used in the medical treatment of chronic bronchitis, cancer and paracetamol intoxication (15). The prevention of biofilm formation and adherence to biomaterials devices is another possible role of NAC (17).
Considering the results previously obtained with these compounds, the purpose of this work was to investigate the possible synergistic effect of farnesol with N-acetylcysteine against S. epidermidis planktonic and biofilm cells.
MATERIALS AND METHODS
Bacterial strains and culture conditions
Two clinical isolates of S. epidermidis, known for their ability to form biofilms, were used in this work: strain 1457 (isolated from an infected central venous catheter) and strain 9142, a known producer of the polysaccharide intracellular adhesin (PIA). All strains were gently provided by Dr. G. B. Pier, Channing Laboratory, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston. Both strains were grown for 18 ± 2 h, at 37ºC and 120 rpm in 30 mL of Tryptic Soy Broth (TSB) (Merck, Darmstadt, Germany). Then the cells were centrifuged (9500 ×g, 5 min, 4ºC), washed twice with a saline solution [0.9% NaCl (Merck, Darmstadt, Germany) in distilled water] and sonicated (Ultrasonic Processor, Cole-Parmer Illinois, USA) (22% amplitude, 10s). The cellular suspensions were adjusted to a final concentration of approximately 1 × 109 cells mL−1, determined by optical density at 640 nm, prior to be used in the subsequent assays.
Planktonic assays
Viability assays were performed in 100 mL Erlenmeyers containing a S. epidermidis cell suspension (~ 2 × 108 cells mL−1) in the presence of farnesol (300 μM) (Sigma, St Louis, USA), NA (NAC 1 × MIC = 4 mg mL−1 and 10 × MIC = 40 mg mL−1) (Sigma, St Louis, USA) and farnesol-NAC. It should be noted that 300 μM farnesol was previously shown to be highly effective against planktonic cells of S. epidermidis (7). The suspensions were incubated for 24 hours, at 37ºC and at 130 rpm. Afterwards, cellular viability was assessed by colony forming units (CFU), while cell activity was determined by the XTT ({2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium hydroxide}) (Sigma, St Louis, USA) reduction assay (12).
CFU enumeration: CFU were obtained as follows: 1000 μL of each cellular suspension after being washed with 0.9% NaCl, were resuspended in 0.9% NaCl, followed by 20 s of sonication at 22 W to homogenize the suspension. This procedure disrupted the cell clumps without impairing cell viability (4). Viable cells were determined by performing 10-fold serial dilutions in saline solution and plating in TSA (Merck, Darmstadt, Germany). Colonies were counted after 24 h incubation at 37ºC.
XTT assay: For XTT assay, aliquots of 200 μL of each cell suspension were collected. The cells were washed with 0.9% NaCl by centrifugation for 10 min at 9500 ×g and 4ºC. The pellet was resuspended in 200 μL of 0.9% NaCl and dispensed in a well of a microtiter plate. Then, 50 μL of a solution containing 200 mg L−1 of XTT and 20 mg L−1 of phenazine methosulphate (PMS) (Sigma, St Louis, USA) were added. The microtiter plates were incubated for 3 h at 37ºC in the dark. The absorbance was measured at 490 nm.
Controls included cells not exposed to farnesol or NAC (positive control) and also cells exposed either to farnesol or to NAC alone. All experiments were carried out in triplicate and repeated three times.
Biofilm assays
Biofilm formation and treatment: Biofilms were formed in 96 well tissue culture plates containing 200 μL of S. epidermidis cell suspension (1 × 106 cells mL−1 ) (1457 and 9142 strains) in TSB supplemented with 0.25% glucose (Merck, Darmstadt, Germany) per well to promote biofilm formation. Plates were incubated for 24 h at 37ºC on an orbital shaker (130 rpm). At the end, planktonic cells were removed carefully, and the biofilm was washed twice with 200 μL of 0.9% NaCl. The biofilms were incubated in fresh nutrient medium containing farnesol (300 μM), NAC (1 × MIC and 10 × MIC) and combination of both. XTT, CFU and crystal violet (CV) assays were performed after 24 hours of exposure to antimicrobial agents (alone and in combination) tested. At time 0 (before exposure to antimicrobial agents) the initial cellular concentration of biofilm (~ 2 × 108 cells mL−1 ) was determined.
XTT assay: The quantification of biofilm cellular activity was assessed through the XTT reduction assay. After exposure to farnesol and NAC, biofilms were washed with 0.9% NaCl. Then, 250 μL of a solution containing 200 mg L−1 of XTT and 20 mg L−1 of PMS were added to each well. The microtiter plates were incubated for 3 h at 37ºC in the dark. The absorbance was measured at 490 nm.
CFU enumeration: CFU were obtained as follows: the planktonic cells were removed carefully and the biofilm was washed twice with 200 μL of 0.9% NaCl. The wells were thoroughly scraped and ressuspended in 1 mL of 0.9% NaCl, followed by centrifugation for 10 min at 9500 ×g. The pellet was resuspended in 0.9% NaCl and washed twice, followed by 20 s of sonication at 22 W to homogenize the suspension. Viable cells were determined by performing 10-fold serial dilutions in saline solution and plating in TSA. Colonies were counted after 24 h incubation at 37ºC.
Crystal Violet assay: CV was used as indicator of total biofilm biomass. For the measurement of this parameter, biofilms were washed with 250 μL of 0.9% NaCl, then 250 μL of methanol (Merck, Darmstadt, Germany) were added and left to act during 15 minutes. Afterwards, methanol was removed and 250 μL of crystal violet 1% (v/v) (Merck, Darmstadt, Germany) were added (5 min). The wells were washed with distilled water and finally, acetic acid 33% (v/v) (Merck, Darmstadt, Germany) was added. The absorbance was measured at 570 nm.
Controls were cells not exposed to farnesol or NAC (positive control), and cells exposed either to farnesol or NAC alone. All experiments were carried out in triplicate and repeated three times.
Scanning Electron Microscopy (SEM)
Biofilms were dehydrated by immersion in increasing ethanol (Merck, Darmstadt, Germany) concentration solutions: 70 (10 min), 95 (10 min) and 100% (20 min) (v/v), having then been placed in a sealed desiccator. Samples were mounted on aluminium strubs with carbon tape, sputter coated with gold and observed with a Field Emission Gun - Scanning Electron Microscope (FEG/ESEM) - Nova Nano SEM 200 from FEI Company.
Three fields were used for image analysis. All photographs were taken at a magnification of × 40 000.
Statistical analysis
The results from all assays were compared by the one-way analysis of variance by applying the Bonferroni and Tukey multiple comparison tests, using the SPSS (Statistical Package for the Social Sciences Inc, Chicago). All tests were performed with 95% confidence level.
RESULTS
Figure 1 presents the effect of farnesol, NAC and the association farnesol-NAC on Staphylococcus epidermidis planktonic cells. NAC at 1 × MIC concentration is less effective than farnesol at 300 μM (p < 0.05) (Fig. 1). The combination of farnesol at 300 μM with NAC at 1 × MIC caused a higher CFU log reduction when compared to each one alone (p < 0.05). This combination resulted into an additional log reduction of 0.5 and 1 for strains 1457 and 9142, respectively (p < 0.05) and relatively to the most effective of both antimicrobial agents tested, ie farnesol at 300 μM. However, NAC at 10 × MIC was more effective than farnesol alone and farnesol and NAC 1 × MIC. After 24 hours, NAC 10 × MIC caused 8 log reduction resulting in total cell death (Fig. 1).
Figure 1.
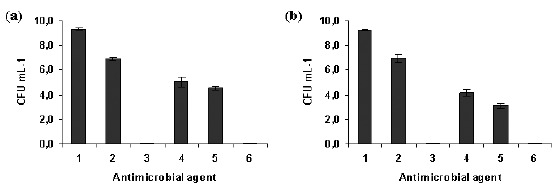
Effect of farnesol and/or NAC on planktonic cells of S. epidermidis 1457 (a) and 9142 (b), after 24 hours of contact with farnesol (300 μM), NAC (4 mg mL−1 and 40 mg mL−1) and farnesol-NAC. Error bars represent standard deviation. Legend: 1- Positive control; 2- NAC 1 × MIC; 3- NAC 10 × MIC; 4- Farnesol 300 µM; 5- Farnesol 300 µM + NAC 1 × MIC; 6- Farnesol 300 µM + NAC 10 × MIC.
Relatively to biofilm cells, although NAC 10 × MIC did not cause total cell death it was the most efficient against S. epidermidis biofilm cells causing a reduction of approximately 4 log (Fig. 2). Conversely to planktonic cells, farnesol and NAC 1 × MIC had a similar effect in biofilms. For strain 1457, NAC 1 × MIC and farnesol worked better together than alone (p < 0.05) (Fig. 2a). There was no synergistic or additional effect when NAC 10 × MIC was combined with farnesol at 300 μM (p < 0.05).
Figure 2.
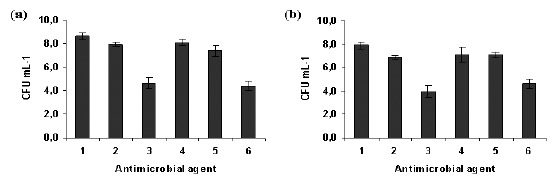
Effect of farnesol and/or NAC on biofilm cells of S. epidermidis 1457 (a) and 9142 (b), after 24 hours of contact with farnesol (300 μM), NAC (4 mg mL−1 and 40 mg mL−1 ) and farnesol-NAC. Error bars represent standard deviation. Legend: 1- Positive control; 2- NAC 1 × MIC; 3- NAC 10 × MIC; 4- Farnesol 300 µM; 5- Farnesol 300 µM + NAC 1 × MIC; 6- Farnesol 300 µM + NAC 10 × MIC.
The results of the XTT reduction assay, indicative of the metabolic activity of cells within the biofilm and CV staining assay, which allows the quantification of the total biofilm biomass, after a 24h treatment with farnesol, NAC, and farnesol+NAC are presented in table 1. These results confirmed the absence of synergy between farnesol and NAC. NAC at 10 × MIC was the antimicrobial agent treatment more efficient against both S. epidermidis strains tested, causing a significant decrease in the metabolic activity of biofilm cells and total biofilm biomass (p < 0.05).
Table 1.
Optical density (absorbance) and confidence interval obtained from XTT and Crystal Violet assays in biofilm cells of S. epidermidis after exposure to farnesol, NAC and farnesol/NAC combination.
| S. epidermidis 1457 | S.epidermidis 9142 | ||||
|---|---|---|---|---|---|
| Condition | XTT | CV | XTT | CV | |
| Positive control | 3.124 ± 0.151 | 2.735 ± 0.280 | 2.314 ± 0.099 | 2.807 ± 0.279 | |
| NAC 1 × MIC | 1.720 ± 0.149 | 2.649 ± 0.232 | 1.452 ± 0.078 | 2.577 ± 0.281 | |
| NAC 10 × MIC | 1.277 ± 0.173 | 1.931 ± 0.117 | 1.124 ± 0.156 | 1.817 ± 0.204 | |
| Farnesol 300 µM | 1.910 ± 0.185 | 2.763 ± 0.250 | 1.715 ± 0.097 | 2.260 ± 0.379 | |
| Farnesol 300 µM + NAC 1 × MIC | 1.928 ± 0.148 | 2.707 ± 0.241 | 1.498 ± 0.161 | 2.132 ± 0.386 | |
| Farnesol 300 µM + NAC 10 × MIC | 1.360 ± 0.106 | 1.868 ± 0.127 | 1.195 ± 0.169 | 1.681 ± 0.290 | |
Representative scanning electron microscopy images of 1457 S. epidermidis biofilms after being exposed to farnesol, NAC and farnesol-NAC are presented on figure 3. These images specifically show the effect on the biofilm matrix and biofilm cell viability, and are in agreement with the results presented above. All biofilms treated with NAC revealed a desintegration of the matrix which is more noticeable for NAC at 40 mg mL−1 (10x MIC). Farnesol seems to have also an effect on biofilm matrix but not as pronounced as NAC.
Figure 3.
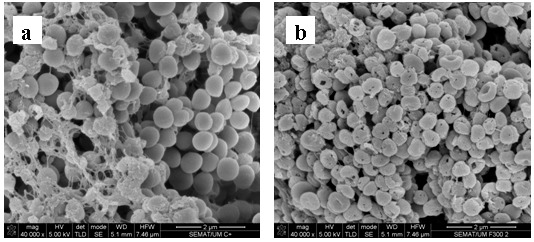
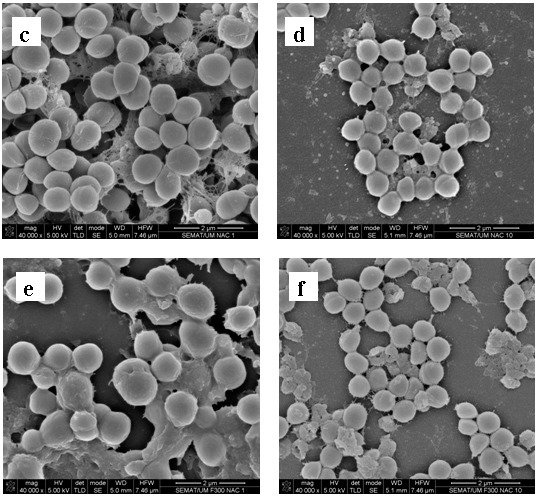
Scanning electron micrographs of 24 h-biofilm of S. epidermidis 1457 after exposure to farnesol, NAC, and the combination of both for 24 h. (a) Positive control; (b) 300 µM farnesol; (c) NAC 1 × MIC; (d) NAC 10 × MIC; (e) Farnesol 300 µM + NAC 1 × MIC; (f) Farnesol 300 µM + NAC 10 × MIC. Magnification × 40 000.
DISCUSSION
In this work, the effect of farnesol, NAC and farnesol-NAC combination against S. epidermidis planktonic and biofilm cells was studied. For that, two good biofilm-forming strains were selected, strains 1457 and 9142 (19). Comparing these two strains, 1457 produces slightly more biofilm than 9142 (19). The biofilm formation ability is due to the formation of PIA homopolymer, which surrounds and connects S. epidermidis cells in biofilm form (16). The extracellular matrix is extremely important for intercellular connection during surface colonization (10) and protection against the host immune system and resistance to antibiotics (1). Figure 3a represents a 48 hours biofilm of S. epidermidis 1457 and shows the thickness of biofilm and the presence of a noticeable amount of biofilm matrix.
N-acetylcysteine (NAC), a potent antioxidant that reduces disulphide bonds linking mucin oligomers, has been widely used as a mucolytic agent for inhalation therapy in patients with chronic bronchitis. NAC has been shown not only to reduce adhesion but also to detach bacterial cells adhered to surfaces and to inhibit bacterial growth in vitro (15). NAC decreases biofilm formation by a variety of bacteria and reduces the production of extracellular polysaccharide matrix, while promoting the disruption of mature biofilm (2).
On the other hand, the principal interaction of farnesol appears to be with the cytoplasmatic membrane (11). Farnesol is a sesquiterpenoid that already demonstrated synergistic effect with another antimicrobial agent (gentamicin) indicating a potential application as an adjuvant therapeutic agent (11). According to previous studies, where farnesol was tested at concentrations ranging from 30 to 300 µM, the last concentration demonstrated to have an antimicrobial effect against S. epidermidis as well as against other bacteria (7, 11).
We hypothesized that the combination of NAC with farnesol could be synergystic in the treatment of S. epidermidis infections as they both act on different components of the biofilm. Our results revealed that additionally to be bactericidal NAC seems also to act against the matrix. In fact, NAC seems to destroy the biofilm matrix resulting in the detachment of cells and thus the biofilm cells become more exposed and susceptible. This high effect against biofilm cells of S. epidermidis must be due in part to the small molecular size of NAC (Molecular Weight = 163.19), which easily penetrates into the biofilm. NAC at 1 × MIC in combination with 300 µM farnesol resulted in a higher antimicrobial effect against planktonic cells of S. epidermidis 1457 and 9142 than both antimicrobial agents alone. Nevertheless NAC alone at 10 × MIC, similarly to biofilms, showed a very high bactericidal effect. Although its very high effect on planktonic cells promoting CFU reductions above 8 log, it is probably more impressive its bactericidal effect on biofilms, which are always very tolerant to the most common antibiotics (7). However, unlike it was expected it did not work in synergy with farnesol at 300 µM against biofilm cells.
Comparatively to planktonic cells, biofilm cells were much more tolerant to the inhibitory effect of farnesol, NAC and farnesol-NAC. As mentioned above, this fact must be due to the protective effect of the matrix. The effect of NAC was concentration dependent. While with NAC at 1 × MIC an average reduction of 2.5 log was observed, NAC 10 × MIC was enought to kill all planktonic cells. However, for biofilm cells this concentration (10 × MIC) only promoted an approximatly 4 log reduction in the number of viable cells within the biofilm, while only 1 log was attained with 1 × MIC.
The peak serum concentration of NAC after a 600 mg oral dose was estimated to be 0.465 mg mL−1 (18). The concentrations of NAC tested in our study (1 × MIC and 10 × MIC, 4 and 40 mg mL−1) are rather higher than those reached in serum when applied by the intravenous or oral route. However, when applied locally it may be possible to obtain concentrations that prevent the formation of biofilms and consequently the adherence of S. epidermidis. (17).
In another study, a concentration of 80 mg mL-1 of NAC was tested in vitro based on preliminary data that showed a dose-response relashionship on planktonic bacteria (2). Based in these results it seems to be feasible the use of 40 mg mL-1in vivo.
In conclusion, NAC at 40 mg mL-1 was the only of the tested treatments that was bactericidal against S. epidermidis cells both in planktonic or in biofilm form. Moreover, although NAC and farnesol have different modes of action, the combination of both has no significant synergistic effect.
ACKNOWLEDGEMENTS
Fernanda Gomes and Pilar Teixeira fully acknowledge the financial support of Fundação para a Ciência e Tecnologia (FCT) through the grants SFRH/BD/32126/2006 and SFRH/BPD/26803/2006, respectively.
REFERENCES
- 1.An Y., Friedman R. Laboratory methods for studies of bacterial adhesion. J. Microbiol. Methods. 1997;30:141–152. [Google Scholar]
- 2.Aslam S., Trautner B.W., Ramanathan V., Darouiche R. Combination of tigecycline and N-acetylcysteine reduces biofilm-embedded bacteria on vascular catheters. Antimicrob. Agents Chemother. 2007;51:1556–1558. doi: 10.1128/AAC.00893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cargill J.S., Upton M. Low concentration of vancomycin stimulate biofilm formation in some clinical isolates of Staphylococcus epidermidis. J. Clin. Pathol. 2009;62:1112–1116. doi: 10.1136/jcp.2009.069021. [DOI] [PubMed] [Google Scholar]
- 4.Cerca N., Martins S., Cerca F., Jefferson K.K., Pier G.B., Oliveira R., Azeredo J. Comparative assessment of antibiotic susceptibility of coagulase-negative staphylococci in biofilm versus planktonic culture as assessed by bacterial enumeration or rapid XTT colorimetry. J. Antimicrob. Chemother. 2005a;56:331–336. doi: 10.1093/jac/dki217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerca N., Martins S., Sillankorva S., Jefferson K.K., Pier G.B., Oliveira R., Azeredo J. Effects of growth in the presence of subinhibitory concentrations of dicloxacillin on Staphylococcus epidermidis and Staphylococcus haemolyticus biofilms. Appl. Environ. Microbiol. 2005b;71:8677–8682. doi: 10.1128/AEM.71.12.8677-8682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derengowski L.S., De-Souza-Silva C., Braz S.V., Mello-De-Sousa T.M., Báo S.N., Kyaw C.M., Silva-Pereira I. Antimicrobial effect of farnesol, a Candida albicans quorum sensing molecule, on Paracoccidioides brasiliensis growth and morphogenesis. Ann. Clin. Microbiol. Antimicrob. 2009;8:13. doi: 10.1186/1476-0711-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes F.I.A., Teixeira P., Azeredo J., Oliveira R. Effect of farnesol on planktonic and biofilm cells of Staphylococcus epidermidis. Curr. Microbiol. 2009;59:118–122. doi: 10.1007/s00284-009-9408-9. [DOI] [PubMed] [Google Scholar]
- 8.Hajdu S., Lassnigg A., Graninger W., Hirschl A.M., Presterl E. Effects of vancomycin, daptomycin, fosfomycin, tigecycline, and ceftriaxone on Staphylococcus epidermidis biofilms. J. Orthop. Res. 2009;27:1361–1365. doi: 10.1002/jor.20902. [DOI] [PubMed] [Google Scholar]
- 9.Hellmark B., Unemo M., Nilsdotter-Augustinsson A., Söderquist B. Antibiotic susceptibility among Staphylococcus epidermidis isolated from prosthetic joint infections with special focus on rifampicin and variability of the rpoB gene. Clin. Microbiol. Infect. 2009;15:238–244. doi: 10.1111/j.1469-0691.2008.02663.x. [DOI] [PubMed] [Google Scholar]
- 10.Hussain M., Hasting J.G.M., White P.J. Isolation and composition of the extracellular slime made by coagulase staphylococci in a chemically defined medium. J. Infect. Dis. 1991;163:534–541. doi: 10.1093/infdis/163.3.534. [DOI] [PubMed] [Google Scholar]
- 11.Jabra-Rizk M.A., Meiller T.F., James C.E., Shirtliff M.E. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2006;50:1463–1469. doi: 10.1128/AAC.50.4.1463-1469.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn D.M., Balkis M., Chandra J., Mukherjee P.K., Ghannoum M.A. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol. 2003;41:506–508. doi: 10.1128/JCM.41.1.506-508.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuroda M., Nagasaki S., Ohta T. Sesquiterpene farnesol inhibits recycling of the C55 lipid carrier of the murein monomer precursor contributing to increased susceptibility to β-lactams in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2007;59:425–432. doi: 10.1093/jac/dkl519. [DOI] [PubMed] [Google Scholar]
- 14.Monzón M., Oteiza C., Leiva J., Amorena B. Synergy of different antibiotic combinations in biofilms of Staphylococcus epidermidis. J. Antimicrob. Chemother. 2001;48:793–801. doi: 10.1093/jac/48.6.793. [DOI] [PubMed] [Google Scholar]
- 15.Olofsson A., Hermansson M., Elwing H. N-acetyl-L-Cysteine affects growth, extracellular polysaccharide production, and bacterial biofilm formation on solid surfaces. Appl. Environ. Microbiol. 2003;69:4814–4822. doi: 10.1128/AEM.69.8.4814-4822.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otto M. Staphylococcus epidermidis- the “accidental” pathogen. Microbiology. 2009;7:555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pérez-Giraldo C., Rodriguez-Benito A., Moran F.J., Hurtado C., Blanco M.T., Gomez-Garcia A.C. Influence of N-acetylcysteine on the formation of biofilm by Staphylococcus epidermidis. J. Antimicrob. Chemother. 1997;39:643–646. doi: 10.1093/jac/39.5.643. [DOI] [PubMed] [Google Scholar]
- 18.Rehman T., Fought J., Solomon R. N-acetylcysteine effect on serum creatinine and cystatin C levels in CKD Patients. Clin. J. Am. Soc. Nephrol. 2008;3:1610–1614. doi: 10.2215/CJN.01560408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sousa C., Teixeira P., Oliveira R. The role of extracellular polymers on Staphylococcus epidermidis biofilm biomass and metabolic activity. J. Basic Microbiol. 2009;49:363–370. doi: 10.1002/jobm.200800196. [DOI] [PubMed] [Google Scholar]
- 20.Vuong C., Kocianova S., Yao Y., Carmody A.B., Otto M. Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo. J. Infect. Dis. 2004;190:1498–1505. doi: 10.1086/424487. [DOI] [PubMed] [Google Scholar]
- 21.Wang C., Li M., Dong D., Wang J., Ren J., Otto M., Gao Q. Role of ClpP in biofilm formationand virulence of Staphylococcus epidermidis. Microbes Infect. 2007;9:1376–1383. doi: 10.1016/j.micinf.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Ziebuhr W., Hennig S., Eckart M., Kränzler H., Batzilla C., Kozitskaya S. Nosocomial infections by Staphylococcus epidermidis: how a commensal bacterium turns into a pathogen. Int. J. Antimicrob. Agents. 2006;28:14–20. doi: 10.1016/j.ijantimicag.2006.05.012. [DOI] [PubMed] [Google Scholar]


