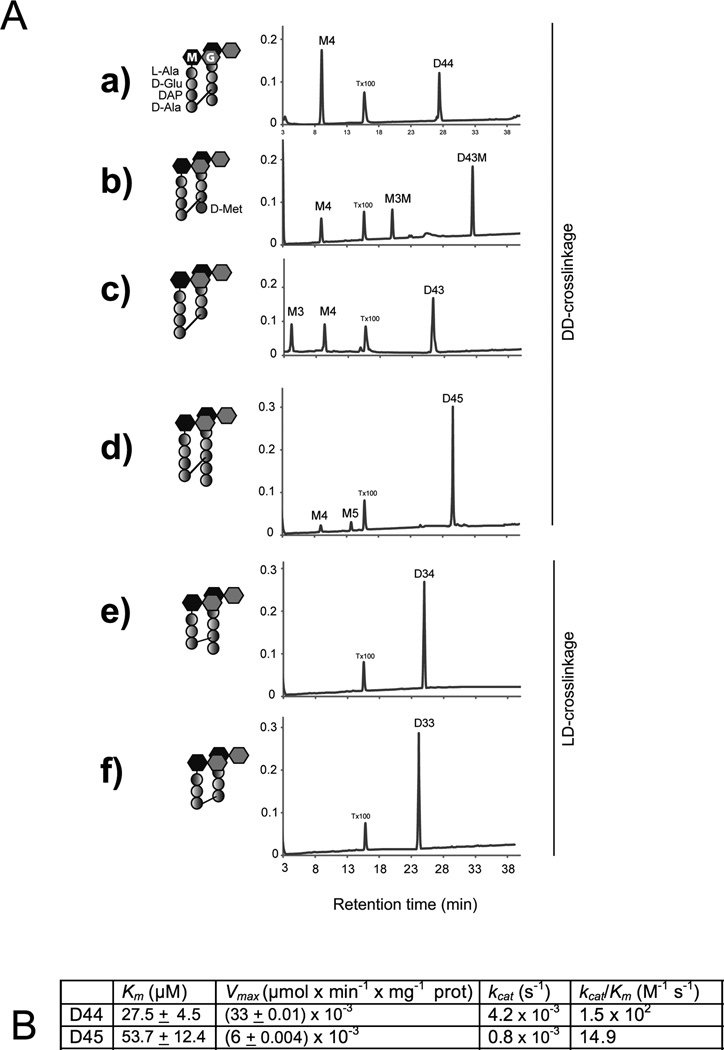
Fig. 6. ShyA substrate preferences.
(A) HPLC analysis of ShyA endopeptidase activities on muropeptides The indicated DD-crosslinked dimers (D44, D43, D43M, D45) and LD-crosslinked dimer muropeptide substrates (D33 and D34) were incubated with 7.8 µM ShyA-His for 3h at 37˙C before HPLC analysis. ShyA endopeptidase activity was assayed by monitoring the appearance of the monomeric compounds M4 (GM-L-Ala-D-Glu-DAP-D-Ala); M3 (GlcNAc-MurNAc-L-Ala-D-Glu-DAP), M5 (GM-L-Ala-D-Glu-DAP-D-Ala-D-Ala) and M3M (GM-L-Ala-D-Glu-DAP-D-Met). Assays were carried out in duplicates and representative HPLC chromatograms are shown. X-axis = A204 (B) Kinetics of ShyA-His D,D-endopeptidase activities. D,D-endopeptidase activity in vitro on D44 and D45 muropeptide substrates was measured as described in experimental procedures. All kinetic constants were calculated using data obtained with 7.8 µM ShyA with various amounts (1–300 µM) of the dimers bis-dissacharide tetratetrapeptide (D44) and bis-dissacharide tetrapentapeptide (D45). The enzymatic reactions were analyzed by HPLC assay as described in experimental procedures. All kinetic constants must be considered apparent values because of the impossibility of calculating initial enzyme velocities by HPLC. Values are means ± standard deviations of experimental triplicates.