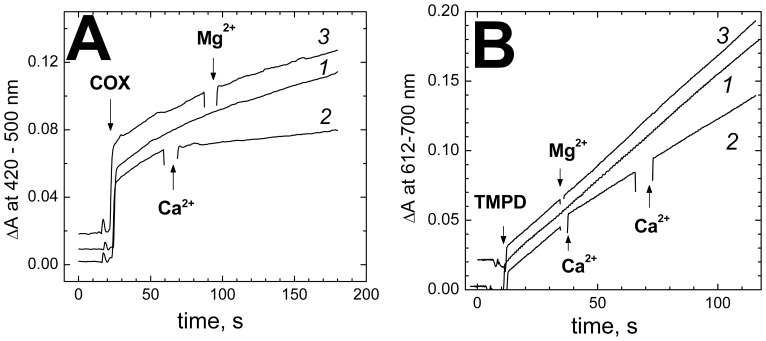
Figure 3. Ca2+ inhibits aerobic oxidation of artificial electron donors by COX.
(A) Oxidation of ferrocyanide. 0.2 mM ferrocyanide in the basic buffer pH 8.2, supplemented with 30 µg/ml of poly-L-lysine to stimulate reaction of COX with ferrocyanide anion [38]. Reaction is initiated by addition of 0.4 µM bovine COX and accumulation of ferricyanide is followed at 420 nm vs the 500 nm reference. Trace 1, control recording with no other additions; trace 2, 200 µM CaCl2 added where indicated; trace 3, 400 µM MgSO4 added instead of CaCl2. The initial upward jump of the traces is due to absorption of the added COX. (B) Oxidation of N,N,N’,N’ – tetrametyl-p-phenylenediamine. 0.15 µM bovine COX in the basic buffer. Where indicated, 0.1 mM reduced TMPD is added, and its oxidation to Wurster’s Blue is followed spectrophotometrically at 612 nm vs the 700 nm reference. Trace 1, control recording with no additions; trace 2, 200 µM CaCl2 added where indicated, note that the second addition does not induce any further inhibition; trace 3, 200 µM Mg2+ added where indicated. The kinetics curves in the panels A and B are displaced arbitrarily on the ordinate axis for clarity.