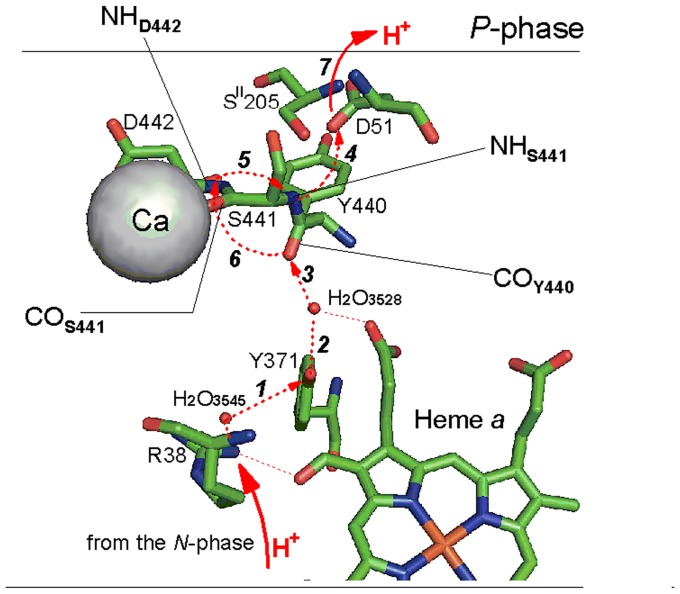
Figure 8. Interaction of Ca2+ with the exit of proton channel H in bovine heart oxidase.
Structure of the exit part of the H-channel is shown based on the oxidized COX crystal structure, PDB entry 1V54. All the groups shown belong to subunit I (polypeptide A) except for SII205 from subunit II (polypeptide B). The scheme gives a scenario of proton transfer combined from refs. [44], [45], [49]. Proton trajectory is depicted by red arrows and sequence of the proton transfer steps is indicated by numbers. Oxidation of heme a by the binuclear site brings about a conformational change that unlocks the H-channel below the heme [46] (cf. [50] for an alternative proposal) and pumped proton arrives to the guanidine group of R38 from the N-aqueous phase (step 1) via the input part of the H-channel (cf. Figure 1A ); it travels further via the R38-bound H2O3545 (that has then to shift closer to Y371), OH group of Y371 and H2O3528 to finally protonate the backbone carbonyl group of Y440 (steps 2–4). The carbonyl function C = OY440 protonated, makes the -NHS441 acidic (imidic acid) and allows for its facile spontaneous deprotonation by carboxylate of D51 (step 5), converting the S441-Y440 peptide bond to the enol form. The protonated state of D51 is then stabilized by multiple hydrogen bonding to SII205 and S441. As proposed in [44], the enol form of the S441-Y440 peptide bond returns to the initial keto form (the notorious proton transfer via the peptide bond S441-Y440 [44], [45], [49]) actually in two steps. First, the deprotonated enolic = NS441 receives proton from the backbone -NH of D442 (step 6, the rate limiting stage of the entire process), which is followed by facile reprotonation of -N- D442 by the protonated backbone C-OHY440 (step 7) returning the latter to the initial carbonyl state. Upon subsequent reduction of heme a, D51 undergoes reorientation associated with loss of the stabilizing hydrogen bonding to SII205 and, hence, decreased proton affinity, so that its carboxylic group releases the proton to the P-phase (step 8). Ca2+ coordinates to the backbone carbonyl oxygen of S441, and also makes a bond with the carboxylate of D442 via intercalated fixed water molecule (hidden behind the Ca ion in this projection of the structure, cf. H2O3544 in Figure 1B and see ref. [14]). As discussed in the text, Ca2+ is expected to inhibit proton transfer through the exit part of the H-channel and, accordingly, to impede the proton transfer-coupled electron transfer by COX.