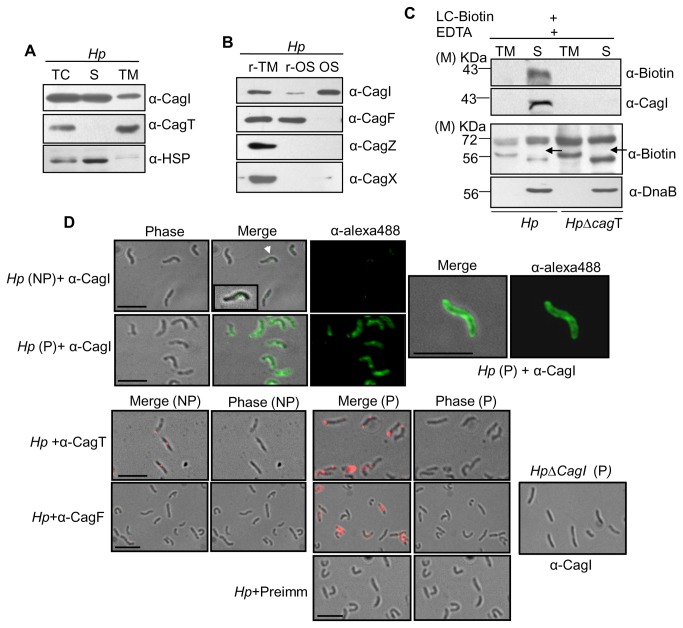
Figure 1. Cellular localization of CagI in H. pylori.
(A) Western blots showing sub-cellular fractionation of wild-type H. pylori. TC, S and TM indicate total-cell lysate, soluble (cytoplasmic/periplasmic) and total membrane fractions respectively. Volume of TM was adjusted corresponding to that of S fraction and then equal volume of each was loaded in to gel. (B) Western blots showing osmotic shock analysis of H. pylori. r-TM, r-OS, and Os stand for residual total membrane, residual osmotic shocked content (cytosolic contents) and osmotic shocked fraction (periplasmic contents) respectively. Antibodies used are marked. (C) Western blots showing selective biotinylation of CagI from biotin labeled wild-type H. pylori (Hp) and HpΔcagT mutant strains. TM and S stand for total membrane fraction and soluble fraction. Antibodies used are marked. M-indicates molecular size standard. Arrow indicates position of non-biotinylated DnaB. (D) Immunofluorescence microscopy showing cellular localization of CagI in wild-type H. pylori (Hp) under permeabilized (P) and non-permeabilized (NP) conditions. H. pylori cells were fixed and permeabilized with 0.2% Triton X-100 and probed with antibodies as indicated. Alexa fluor 488 (green colour) and Alexa fluor 594 (red colour) conjugated secondary antibodies were used for signal generation and finally examined by immunofluorescence microscopy. Permeabilized HpΔcagI cells are showing specificity of α-CagI antibody. Pre-immune serum was used as control antibody. Scale bars indicate 5µM.