Fig. 1.
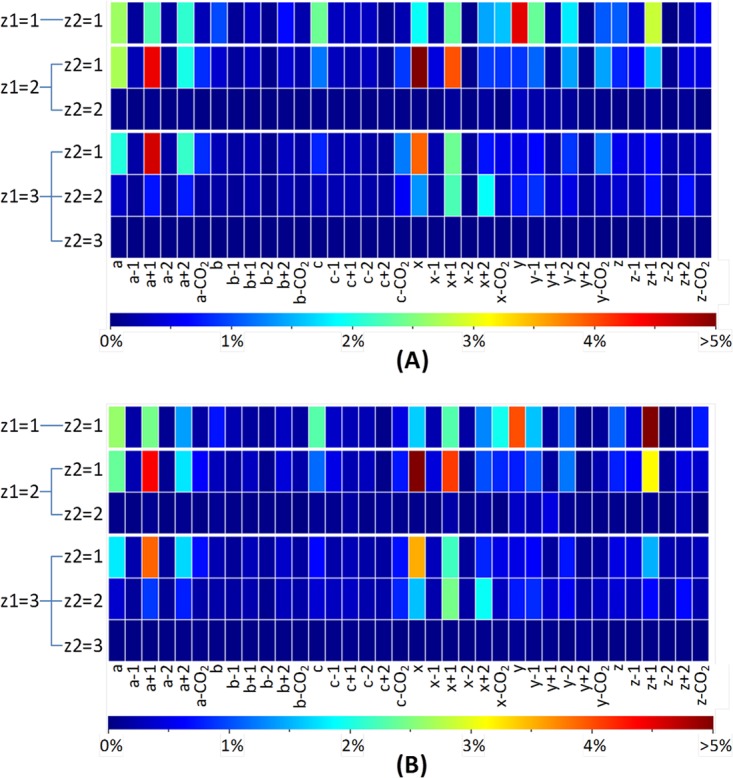
The fragmentation behavior of tryptic HeLa and Halo peptide anions as determined via UVPD from the optimized search algorithm in MassMatrix: ion intensities of various product ions normalized by total ion intensities of the spectrum (after precursor and intact charge-reduced peak filtering) for tryptic peptide matches with FDR < 1% from (A) Halo and (B) HeLa proteome samples. For the Halo proteome sample, 14,802 peptide spectral matches (PSMs) from 3663 unique peptides were identified. Among them, 726 PSMs from 328 unique peptides were singly charged, 12,112 PSMs from 3121 unique peptides were doubly charged, and 1964 PSMs from 617 unique peptides were triply charged. For the HeLa proteome sample, there were 7886 PSMs from 2349 unique peptides identified. Among them, 232 PSMs from 121 unique peptides were singly charged, 5972 PSMs from 1991 unique peptides were doubly charged, and 1682 PSMs from 568 unique peptides were triply charged. Only one representative PSM for each unique peptide with a given charge state was used in plotting the heat maps.
