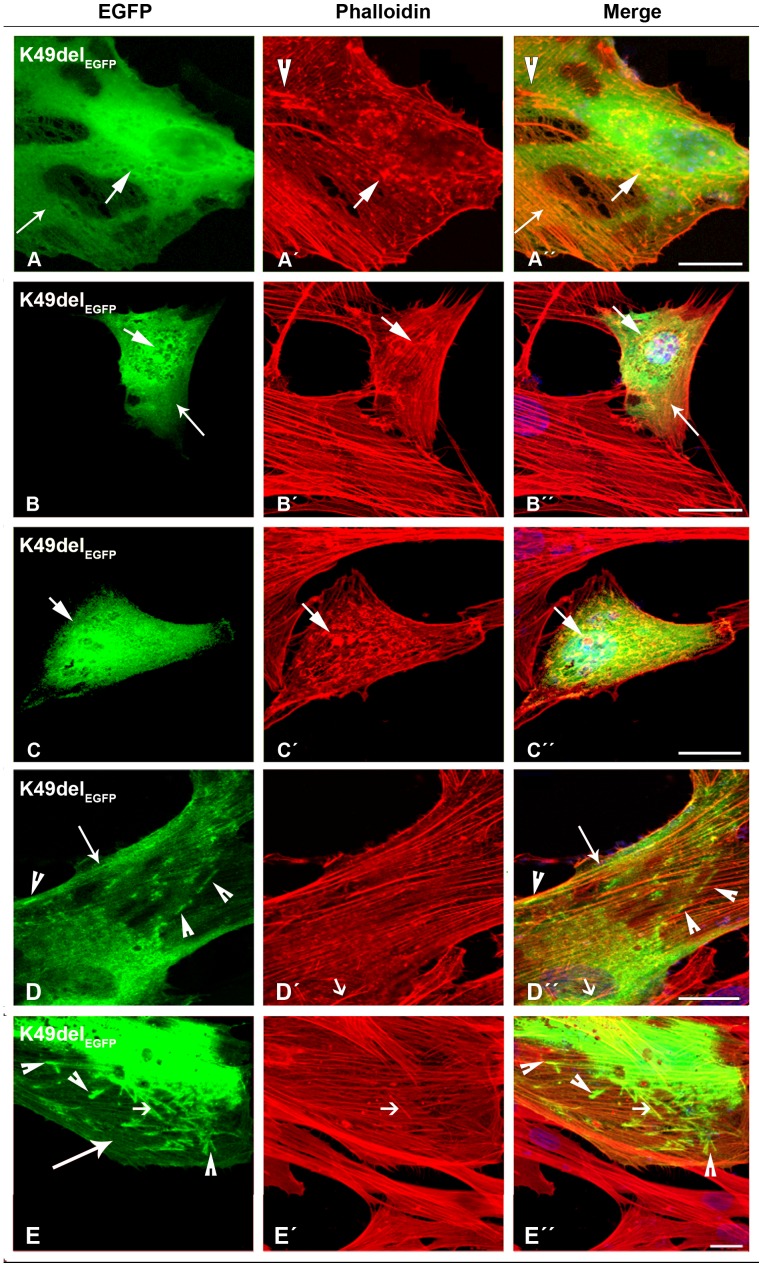
Figure 3. The expression of K49del-β-TMEGFP in human cells.
(A–D) The K49del-β-TMEGFP mutant was transfected in human myoblasts and (E) myotubes and labeled with TRITC-phalloidin (red) and DAPI (blue) to highlight cell nuclei. The K49del-β-TM co-localised with cytoplasmic and nuclear aggregates of endogenous actin, detectable with phalloidin labeling (A–C”; short arrows). It produced clouds around the nucleus (A and D) but was also incorporated into stress fibres and filamentous lamellipodia (A and A”, B and B”, and D and D”; long arrows). In addition, it induced thickened filamentous structures of endogenous actin (A’–A”; arrow heads). The K49del-β-TM produced small, long rod-shaped intranuclear structures. A subset of aggregates labeled with phalloidin but was not detectably composed of EGFP-tagged mutant K49del-β-TM (D’–D”; short arrows). Frequently, cytoplasmic thickened filamentous structures with no co-localisation of F-actin were found in myoblasts transfected with K49del-β-TM (D and D”; arrow heads). The K49del-β-TMEGFP mutant produced rod-shaped filamentous actin, cytoplasmic aggregates and cloud patterns in human myotubes (E). The rod-shaped structures were labeled with phalloidin, indicating co-localisation with F-actin (E–E”; short arrows). Cytoplasmic thickened filamentous structures were also observed (E and E”; arrow heads). The K49del-β-TMEGFP mutant was also incorporated into filamentous actin (E; long arrow). Confocal microscopy was performed using a Zeiss LSM 510 Meta confocal microscope or an LSM 700 inverted Axio Observer.Z1 microscope. Scale bar = 10 µm.