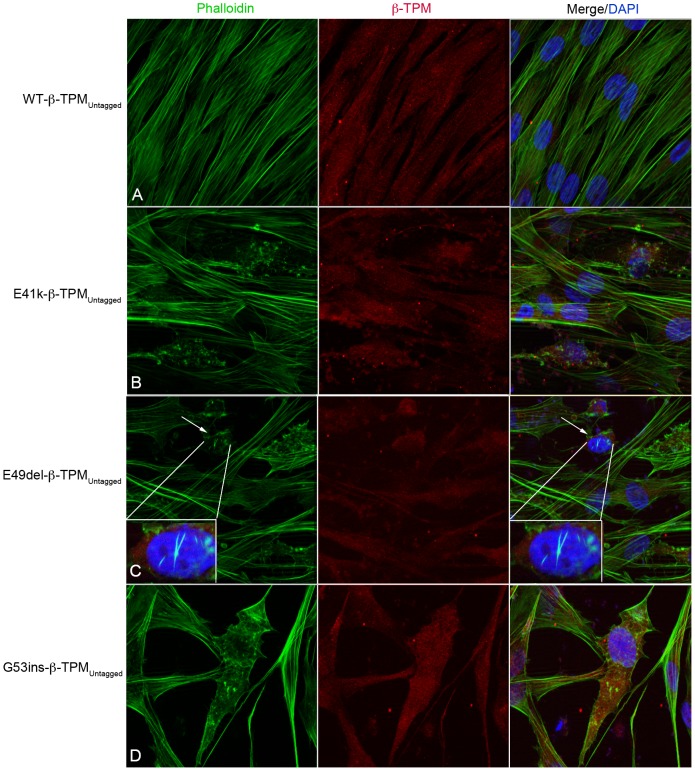
Figure 8. Untagged β-TM constructs form abnormal aggregates in human myoblasts.
Human myoblasts transfected with untagged WT-, E41K-, K49del- and G53ins-β-TM constructs and labeled with phalloidin (green) and β-TM (red) and DAPI (blue) to highlight cell nuclei. Stress fibres appeared well aligned in human myoblasts transfected with WT-β-TM (A). Abnormal aggregates were observed in human myoblasts transfected with untagged E41K-, K49del- and G53ins-β-TM constructs (B–D). Intranuclear rod-shaped aggregates labeled by phalloidin are detected in human myoblasts transfected with the untagged K49del-β-TM construct, demonstrating that intranuclear aggregation is an inherent property of K49del mutation and does not result from EGFP-tagging (C; arrows).