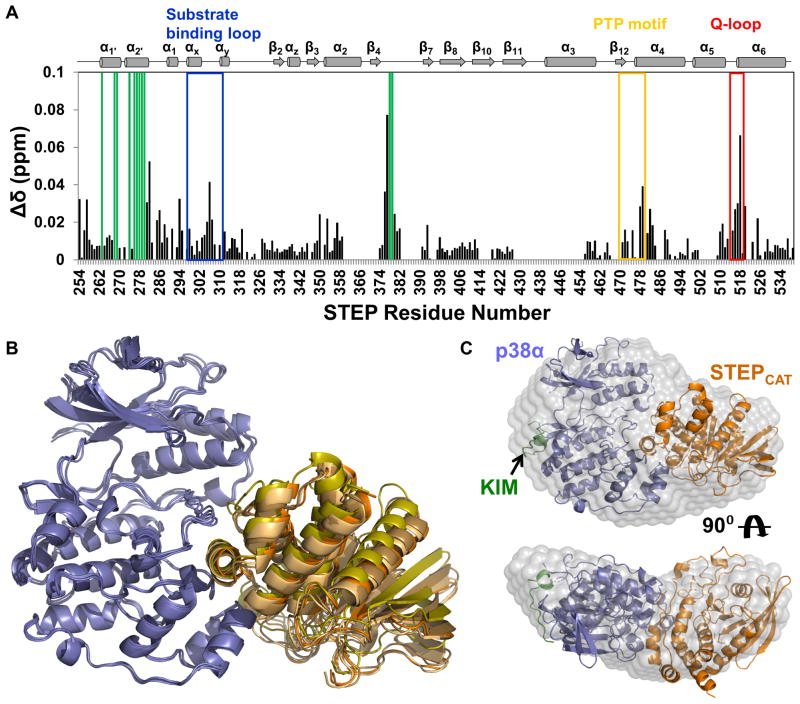
Figure 4. STEPCAT binds to the p38α activation loop and MAPK-insert primarily through helix α-2′.
(A) Histograms showing the combined 1H/15N CSP vs. STEP residue for the interaction of p38α with [2H,15N]-STEP. (B) Alignment of the 4 lowest energy HADDOCK structures of the p38α:STEPCAT complex. p38α is colored blue and STEPCAT is colored in shades of orange. (C) Superposition of the lowest energy HADDOCK p38α:STEPCAT complex structure with the experimental SAXS molecular envelope of the p38α:STEP resting-state complex; p38α (blue), KIM (green) and STEPCAT (orange). See also Figure S4.