Fig. 1. Schematic representation of translational control by eIF2α kinases.
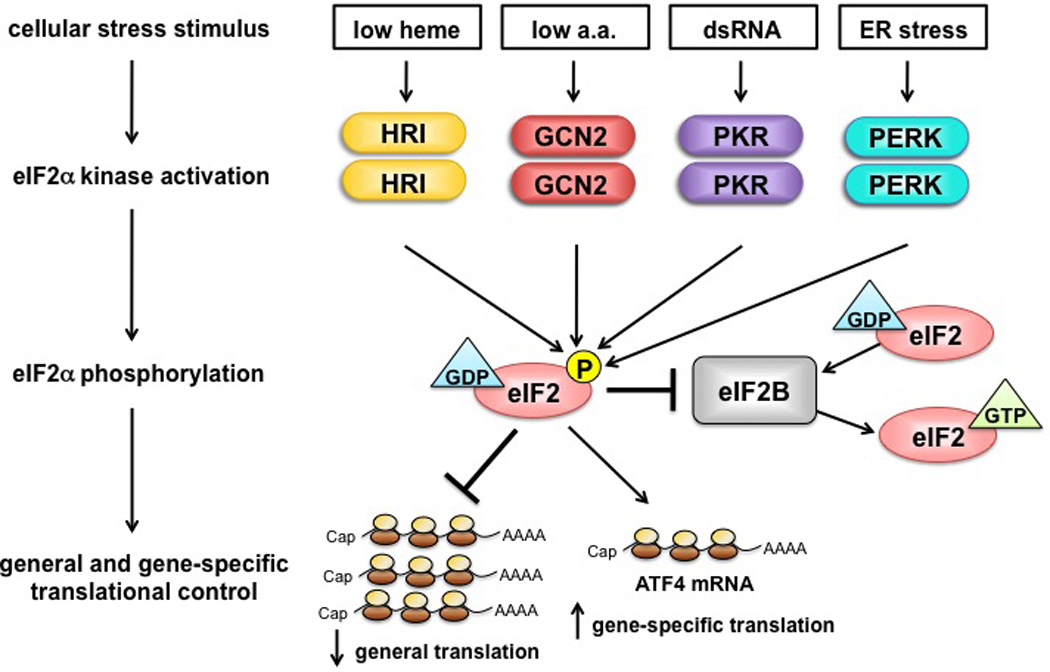
In response to distinct cellular stress stimuli, four eIF2α kinases become activated and phosphorylate the α subunit of eIF2. The guanine nucleotide exchange factor eIF2B catalyzes the exchange of inactive GDP for active GTP-bound eIF2, a process required for new rounds of translation initiation. Phosphorylation of eIF2 inhibits eIF2B activity, which blocks GDP/GTP-exchange, resulting in the reduction of general translation and the stimulation of gene-specific translation of uORF-containing mRNAs (for example, ATF4).
