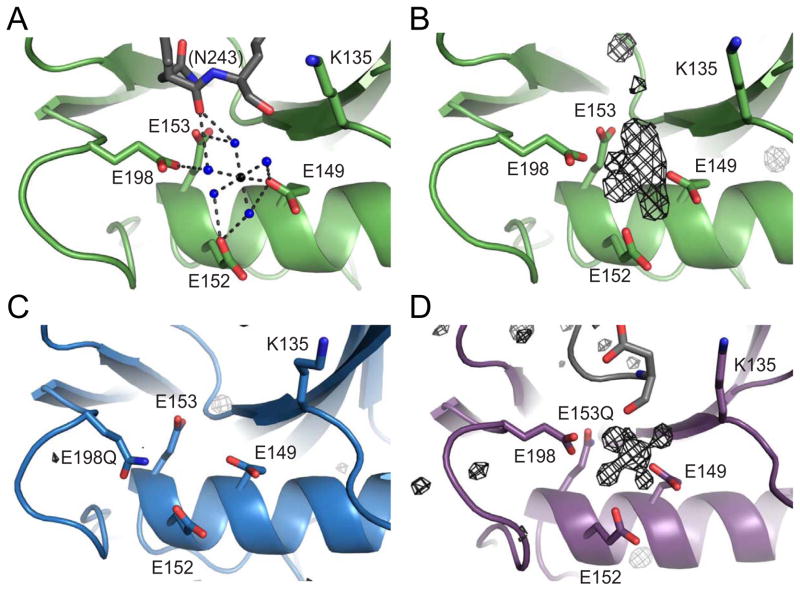
Figure 1. Mg2+ coordination in the Dcp2 catalytic domain.
(A) Ribbon diagram of Mg2+ coordination in the wild-type S. cerevisiae Dcp2 catalytic Nudix domain at the catalytic helix. Four conserved glutamates (E149, E152, E153, E198) and K135 are shown as sticks in green. Water molecules are shown as blue spheres, and the Mg2+ is shown as a black sphere. Colored in grey is the backbone carbonyl of N243 in a symmetry related molecule. Distances between atoms are shown in Å.
(B) Fo−Fc difference electron density map of wild-type Dcp2 depicted as black mesh at an I/σ cutoff of 2.5.
(C) Fo−Fc difference electron density map for the E198Q mutant at an I/σ cutoff of 2.0.
(D) Fo−Fc difference electron density map of E153Q mutant at an I/sigma cutoff of 3.0.