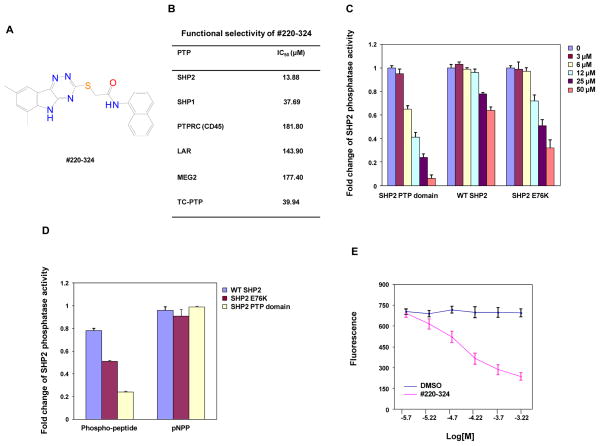
Figure 1. #220–324 selectively inhibited the phosphatase activity of SHP2.
(A) Chemical structure of #220–324. (B) Phosphatase assays were carried out using the indicated phosphatases as enzymes and the phospho-insulin receptor peptide as a substrate in the presence of various concentrations of #220–324, as described in Materials and Methods. Experiments were performed three times with similar results obtained. Representative results from one experiment are shown. (C) PTP activities of the SHP2 PTP domain, WT full length SHP2, and full length SHP2 E76K were determined in the absence or presence of #220–324 at the indicated concentrations using the in vitro phosphatase assay. Experiments were repeated three times. Similar results were obtained in each. Data shown are mean±S.E.M. from one experiment. (D) Inhibitory effects of #220–324 (25 μM) on the enzymatic activities of the SHP2 PTP domain, WT SHP2, and SHP2 E76K were determined using the phospho-insulin receptor peptide or pNPP as the substrates. Experiments were repeated three times. Similar results were obtained in each. Data shown are mean±S.E.M. from one experiment. (E) Fluorescence titration of SHP2 was performed by increasing the concentrations of #220–324 while maintaining the SHP2 protein concentration. The fluorescence is plotted against the log concentration in mol/L (Log [M]) for the test compound. Experiments were repeated twice. Similar results were obtained in each. Data shown are mean±S.E.M. from one experiment.