Abstract
In vitro fruiting bodies were produced from ten different isolates of Cordyceps militaris EFCC C-5736, EFCC C-5941, EFCC C-5976, EFCC C-6040, EFCC C-6849, EFCC C-7268, EFCC C-7342, EFCC C-7992, EFCC C-8027 and EFCC C-8549. Single ascospores were isolated from in vitro grown fruiting bodies and used for fruiting body production in brown rice medium by both intra-strain crossing and out-crossing. Length and dry wt. of stromata grown in vitro were measured. Strains producing highest dry wt. of stromata were selected. Both intra-strain crossings and inter-strain crossings of single ascospore strains were found to produce profuse fruiting bodies of C. militaris.
Keywords: Biological efficiency, Cordyceps militaris, Mating type, Inter-strain crossing, Intra-strain crossing
Recently, Cordyceps species (Clavicipitaceae, Hypocreales, Ascomycota) have been searched for bio-active compounds by biochemists and pharmacologists to cure different diseases. Cordyceps species are specially known as medicinal mushrooms in East Asian countries, including Korea. Among Cordyceps species, C. militaris is one of the vigorously studied species for the pharmacological values such as immunomodulatory, immunoenhancing and anti-tumor activities (Liu et al., 1997; Yoo et al., 2004; Zhao et al., 2000) and antioxidant, anti-inflammatory, antiangiogenic and antinociceptive effects (Won and Park, 2005).
Bio-active compounds such as cordycepin and exopolysaccharides have been extracted from mycelium of Cordyceps militaris in broth culture. For higher production of cordycepin and exopolysaccharides, different nutritional factors have been studied in broth culture of C. militaris (Kim and Yun, 2005; Mao and Zhong, 2006). The limitation for the further production of bio-active compounds from fruiting bodies of Cordyceps species is due to the extreme difficulty in their culture and in vitro production of fruiting bodies. Apart from the submerged culture of mycelium, research on fruiting body production of C. militaris on various media is going on. Recently, in vitro stromata production of C. militaris was successfully established in brown rice medium (Choi et al., 1999; Sung, 1996; Sung et al., 1999, 2002). Other researchers have successively grown C. militaris fruiting bodies on alternative insect pupae (Harada et al., 1995; Sato and Shimazu, 2002). But, unstable fruiting body production poses a serious problem for large scale extraction of bio-active compounds from stromata of C. militaris (Shrestha et al., 2004b; Sung, 1996). More recently, bipolar heterothallism was shown during fruiting body production of C. militaris by inoculating single spore strains in pair-wise combinations in rice pupae medium (Shrestha, 2003; Shrestha et al., 2004a, 2005a, b). Relationship between colony pigmentation and mating type was also investigated by crossing strains with different pigmentation types (Shrestha et al., 2005a).
In previous studies, strains of few specimens of C. militaris were used for fruiting body production. In this study, isolates derived from a large number of specimens were used to select potential strains for higher stromata production. Similarly, inter-strain crossing was also made using strains derived from different isolates for fruiting body production and compared with those of intra-strain crossings. Both intra-strain crossings and inter-strain crossings among strains could produce large number of stromata in brown rice medium.
Materials and Methods
Fungal isolates
Multi-ascospore isolates were obtained from ten different specimens of C. militaris and preserved in Entomopathogenic Fungal Culture Collection (EFCC), Kangwon National University, Chuncheon, Korea. Details of specimens are given in Table 1. Ascospores were discharged in 2% water agar (WA) from each specimen soon after their collection. Agar blocks containing mass of freshly discharged as cospores were transferred to Sabouraud Dextrose agar plus Yeast Extract (SDAY; dextrose 20 g, peptone 5 g, yeast extract 5 g and agar 18 g per 1000 ml; pH 5.6) agar plates for growth of mycelium. These mycelial cultures were designated as multi-ascospore isolates of C. militaris.
Table 1.
Specimens of Cordyceps militaris used in this experiment
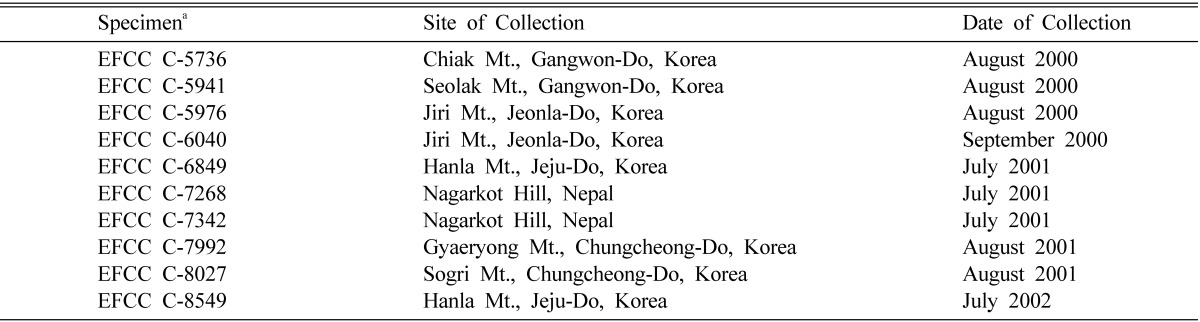
aEFCC, Entomopathogenic Fungal Culture Collection, Kangwon National University, Korea.
Fruiting body production from multi-ascospore isolates of C. militaris
Mycelial discs (5 mm in diameter) were cut with the help of a cork borer from multi-ascospore isolates EFCC C-5736, EFCC C-5941, EFCC C-5976, EFCC C-6040, EFCC C-6849, EFCC C-7268, EFCC C-7342, EFCC C-7992, EFCC C-8027 and EFCC C-8549 of C. militaris and inoculated in SDAY broth. The inoculated broths were incubated in a rotary shaker at 120 rpm for 3~4 days at 25℃. The fruiting medium, commonly known as rice pupae medium, was prepared by mixing brown rice 60 g and silkworm pupae 10 g in 60 ml of distilled water in a 1000 ml Polypropylene (PP) mushroom fruiting bottle. The mixture was sterilized at 121℃ for 20 min. The broth cultures were inoculated in separate rice pupae media and incubated at 20 ± 1℃ under high humidity (70~90%) and continuous light (500~1000 lux) conditions for 50 days.
Preparation of single ascospore strains
Single ascospores were isolated from the in vitro stromata grown in rice pupae medium. For single ascospore isolation, stromata were detached from rice pupae medium and attached to the innerside of lids of Petri dishes containing WA. Single ascospores were picked up following the method of Shrestha et al. (2004a, 2005a, b) and inoculated on fresh SDAY agar plates.
Selection of strains through intra-strain crossing by hyphal anastomosis
Six single ascospores were isolated from stromata of each isolate EFCC C-5736, EFCC C-5941, EFCC C-5976, EFCC C-6040, EFCC C-6849, EFCC C-7992, EFCC C-8027 and EFCC C-8549 were numbered as C-5736-1~C-5736-6, C-5941-1~C-5941-6, C-5976-1~C-5976-6, C-6040-1~C-6040-6, C-6849-1~C-6849-6, C-7992-1~C-7992-6, C-8027-1~C-8027-6 and C-8549-1~C-8549-6, respectively. Intra-strain crossings were made among strains of the same isolate in rice pupae media following the method of Shrestha et al. (2004a). To prepare inoculum for inoculating rice pupae medium, mycelial discs (5 mm in diameter) were cut from the strains and were inoculated in individual SDAY broths. After inoculation in rice pupae medium in pair-wise combination, the inoculated PP bottles were incubated at 20 ± 1℃ under high humidity (70~90%) and continuous light (500~1000 lux) conditions for 50 days. Mating types of the strains were determined on the basis of perithecial stromata formation following the method of Shrestha et al. (2004a). Each crossing was inoculated in PP bottles in triplicate. The length of stromata was measured. Similarly, stromata were harvested from the rice pupae medium and dried at 60℃ in a dry oven till the constant dry wt. was obtained. The average of length and dry wt. of fruiting bodies were calculated. The best strains were selected based on the dry wt. of fruiting bodies.
Selection of strains through inter-strain crossing by hyphal anastomosis
Single ascospore strains of different isolates having opposite mating types such as C-5736-1 and C-5736-4, C-5941-1 and C-5941-5, C-6040-1 and C-6040-6, C-7992-2 and C-7992-4 and C-8549-2 and C-8549-4 were inter-strain crossed (Table 9). Preparations of liquid inoculum and rice pupae medium, and production of fruiting bodies were same as mentioned above. Each inter-strain crossing was inoculated in three PP bottles in triplicate. Average of length and dry wt. of fruiting bodies were calculated as mentioned above. The best strains were selected based on the dry weights of fruiting bodies.
Table 9.
Perithecial stromata formation by inter-strain crossing of single ascospore strains of Cordyceps militaris
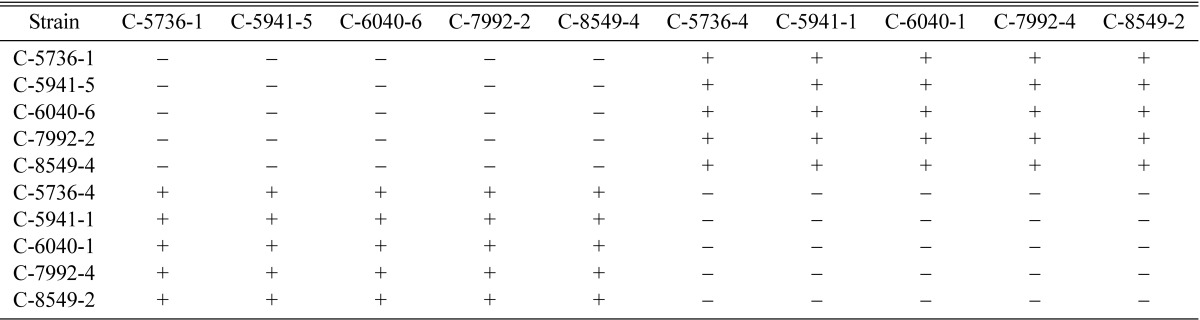
+ indicates perithecial stromata formation, - indicates no or non-perithecial stromata formation.
Results and Discussion
Fruiting body production from multi-ascospore isolates of C. militaris
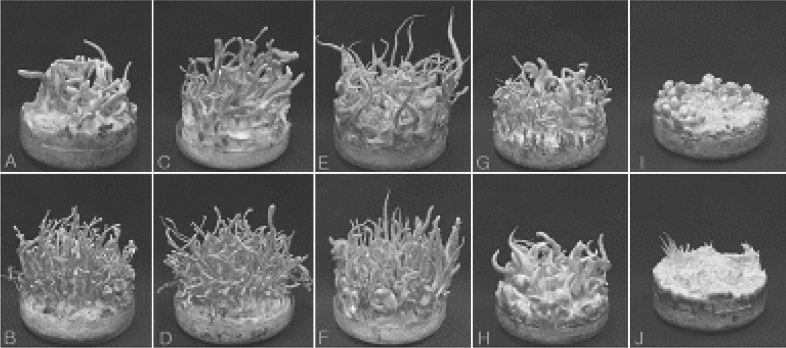
Multi-ascospore isolates EFCC C-5736, EFCC C-5941, EFCC C-5976, EFCC C-6040, EFCC C-6849, EFCC C-7992, EFCC C-8027 and EFCC C-8549 produced fertile fruiting bodies, i.e., perithecial stromata, in rice pupae medium (Fig. 1a~1h). However, fruiting bodies produced from isolates EFCC C-5736 and EFCC C-8027 were covered by white mycelium (Fig. 1a, 1h). The remaining isolates EFCC C-7268 and EFCC C-7342 produced only stromata primordia and could not grow further (Fig. 1I, 1J). The variation in fruiting body production of C. militaris is similar to previous reports (Sung, 1996; Shrestha et al., 2004b). Multi-ascospore isolates are practically simple to make cultures, but produce variable fruiting bodies. We assume that multi-ascospore isolates do so because they consist of a mixture of ascospores having different potentialities of fruiting body production.
Fig. 1.
Fruiting body produced from multi-ascospore isolates of Cordyceps militaris after 50 days of growth in brown rice medium. A, EFCC C-5736; B, EFCC C-5941; C, EFCC C-5976; D, EFCC C-6040; E, EFCC C-6849; F, EFCC C-8549; G, EFCC C-7992; H, EFCC C-8027; I, EFCC C-7268 and J, EFCC C-7342.
Intra-strain crossing by hyphal anastomosis among single ascospore strains and production of fruiting bodies
Mating types of single ascospore strains of EFCC C-5736, EFCC C-5941, EFCC C-6040, EFCC C-7992 and EFCC C-8549 could be identified through intra-strain crossing on the basis of perithecial stromata formation (Tables 2~6). In case of EFCC C-5736 single ascospore strains, C-5736-1, C-5736-3 and C-5736-6 were mating compatible with C-5736-2, C-5736-4 and C-5736-5, but not among themselves (Table 2). Similarly, C-5941-1, C-5941-2, C-5941-3 and C-5941-4 were mating compatible with C-5941-5 and C-5941-6 among single ascospore strains of EFCC C-5941 (Table 3). Out of six single ascospore strains of EFCC C-6040, only one strains (C-6040-6) was mating compatible with the rest five strains (C-6040-1~C-6040-5) (Table 4). Among EFCC C-7992 single ascospore strains, C-7992-1, C-7992-2 and C-7992-5 were mating compatible with C-7992-3, C-7992-4 and C-7992-6 (Table 5). Among EFCC C-8549 single ascospore strains, C-8549-1 and C-8549-4 were mating compatible with C-8549-2, C-8549-3, C-8549-5 and C-8549-6 (Table 6). But, no perithecial stromata could be formed from single ascospore strains of EFCC C-5976 (Table 7), EFCC C-6849 (similar to EFCC C-5976) and EFCC C-8027 (similar to EFCC C-5976) by intra-strain crossing. It was supposed that all the single ascospore strains of EFCC C-5976, EFCC C-6849 and EFCC C-8027 were of the same mating type and hence no mating compatibility was observed. From the above experiment, single ascospore strains could be separated into two groups depending upon their mating types, as reported earlier (Shrestha et al., 2004a, 2005a, b).
Table 2.
Perithecial stromata formation from single ascospore strains of Cordyceps militaris EFCC C-5736
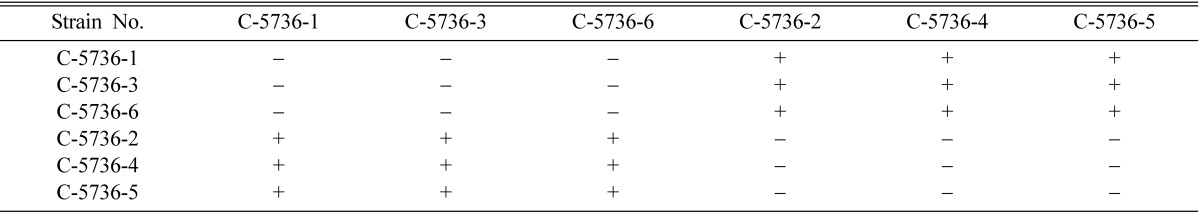
+ indicates perithecial stromata formation, - indicates no or non-perithecial stromata formation.
Table 6.
Perithecial stromata formation from single ascospore strains of Cordyceps militaris isolate EFCC C-8549
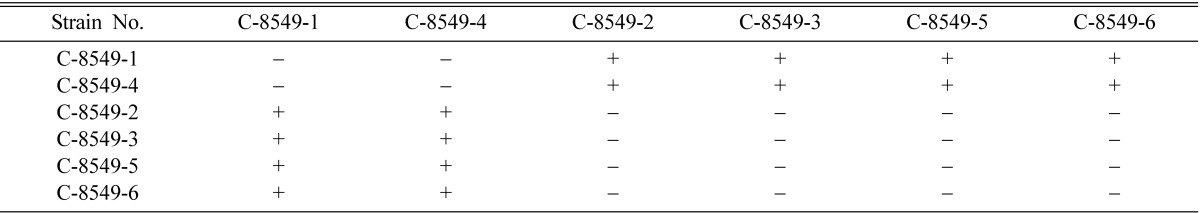
+ indicates perithecial stromata formation, - indicates no or non-perithecial stromata formation.
Table 3.
Perithecial stromata formation from single ascospore strains of Cordyceps militaris EFCC C-5941
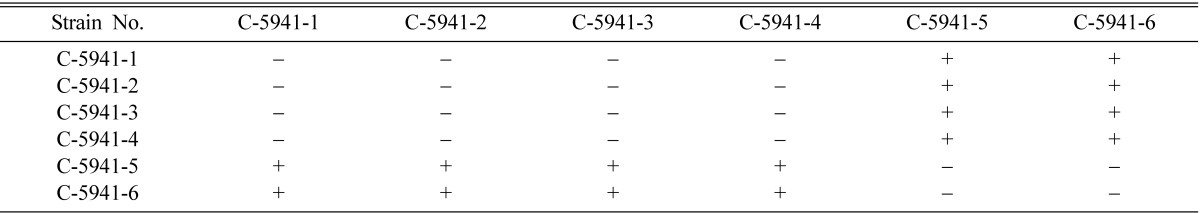
+ indicates perithecial stromata formation, - indicates no or non-perithecial stromata formation.
Table 4.
Perithecial stromata formation from single ascospore strains of Cordyceps militaris EFCC C-6040
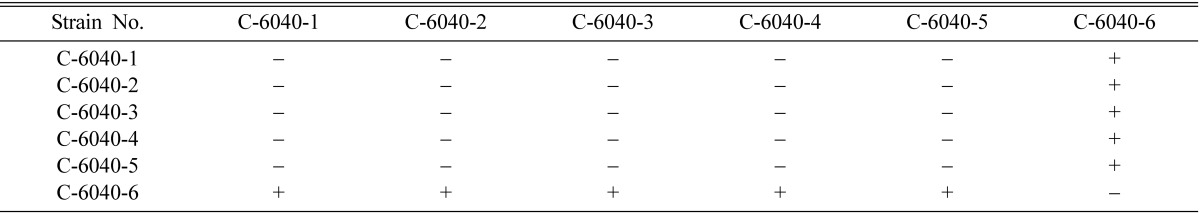
+ indicates perithecial stromata formation, - indicates no or non-perithecial stromata formation.
Table 5.
Perithecial stromata formation from single ascospore strains of Cordyceps militaris EFCC C-7992
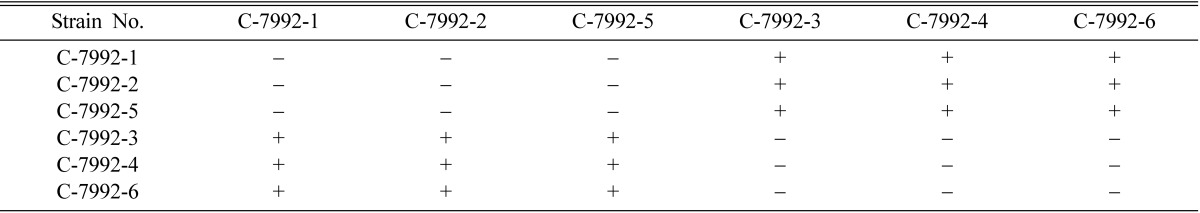
+ indicates perithecial stromata formation, - indicates no or non-perithecial stromata formation.
Table 7.
Perithecial stromata formation from single ascospore strains of Cordyceps militaris EFCC C-5976
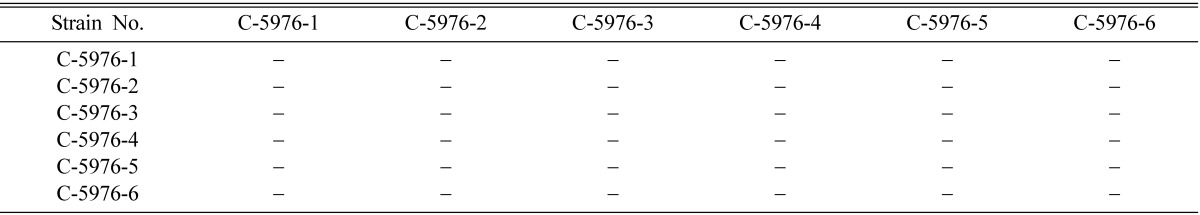
- indicates no or non-perithecial stromata formation.
In order to identify the mating type of single ascospore strains of EFCC C-5976, EFCC C-6849 and EFCC C-8027, strains C-5976-2 C-6849-5 and C-8027-6 were inter-strain crossed with opposite mating type strains C-5736-1 and C-5736-4. Strain C-5736-1 was found mating compatible with C-5976-2, C-6849-5 and C-8027-6 and produced perithecial stromata, whereas strain C-5736-4 was same as C-5976-2, C-6849-5 and C-8027-6 in mating type, hence no perithecial stromata were formed (Table 8).
Table 8.
Determination of mating type of C-5976-2, C-6849-5 and C-8027-6 strains by crossing with tester strains C-5736-1 (mating type A) and C-5736-4 (mating type B)
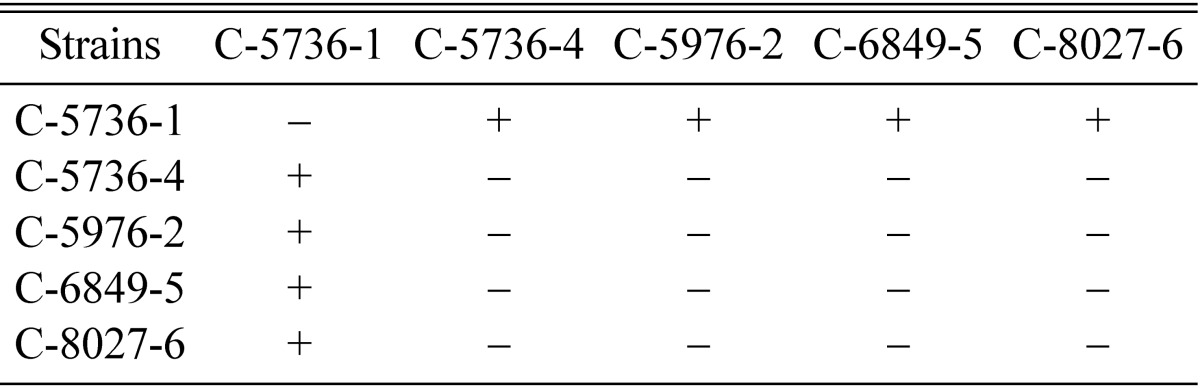
+ indicates perithecial stromata formation, - indicates no or non-perithecial stromata formation.
In order to designate opposite mating type strains, inter-strain crossing was made among strains C-5736-1, C-5736-4, C-5941-1, C-5941-5, C-6040-1, C-6040-6, C-7992-2, C-7992-4, C-8549-2 and C-8549-4 (Table 9). From inter-strain crossing, strains C-5736-1, C-5941-5, C-6040-6, C-7992-2 and C-8549-4 were found to be of the same mating type, whereas remaining strains C-5736-4, C-5941-1, C-6040-1, C-7992-4 and C-8549-2 were found to be of opposite mating type on the basis of perithecial stromata formation (Table 9). Strain C-5736-1 was designated as mating type A whereas the opposite mating type strain C-5736-4 was designated as B. Among total forty-eight single ascospore strains, eleven were found to be of mating type A, whereas thirty seven were found to be of mating type B (Table 10).
Table 10.
Mating types of single ascospore strains of Cordyceps militaris
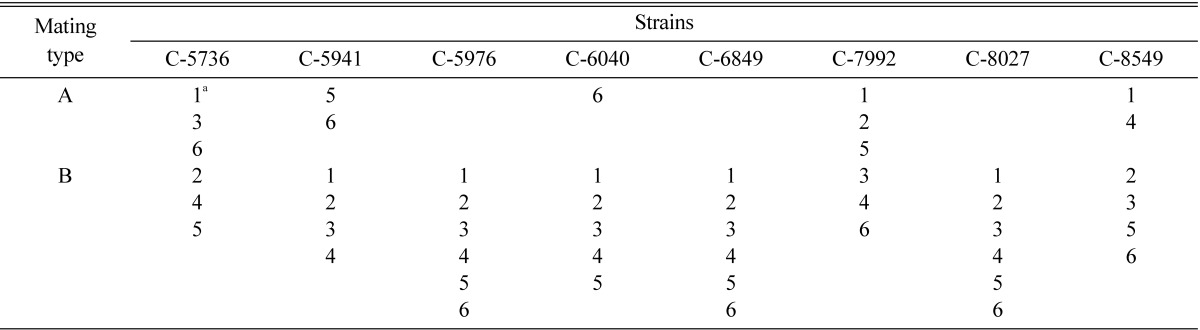
aSingle ascospore strain number.
Selection of strains through intra-strain crossing by hyphal anastomosis
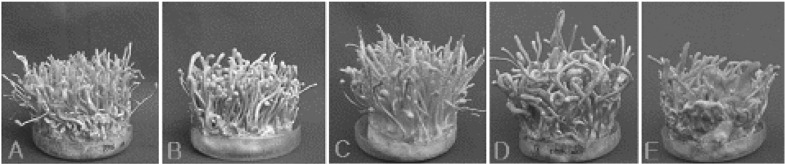
Among EFCC C-5736 strains, the combination C-5736-1 × C-5736-4 produced the highest dry wt. (8.85 ± 0.14 g) of fruiting bodies (Fig. 2A). In case of EFCC C-7992 strains, C-7992-2 × C-7992-4 produced the highest dry wt. (8.46 ± 0.14 g) of fruiting bodies (Fig. 2D). Among different sister-crossings of EFCC C-8549 strains, the combination C-8549-2 × C-8549-4 produced the highest dry wt. (8.23 ± 0.22 g) of fruiting bodies (Fig. 2E). In case of EFCC C-6040 strains, C-6040-1 × C-6040-6 produced the highest dry wt. (7.86 ± 0.16 g) of fruiting bodies (Fig. 2C). Similarly, among strains of EFCC C-5941, the combination C-5941-1 × C-5941-5 produced the highest dry wt. (6.53 ± 0.20 g) of fruiting bodies (Fig. 2B). Although dry wt. of fruiting bodies indicated the amount of fruiting bodies produced from each cross, but the lengths of the fruiting bodies differed from each other. The cross C-6040-1 × C-6040-6 produced the longest stromata of 83 ± 5 mm (Fig. 2C), followed by C-5736-1 × C-5736-4 (67 ± 6 mm, Fig. 2A), C-7992-2 × C-7992-4 (63 ± 6 mm, Fig. 2D), C-8549-2 × C-8549-4 (48 ± 7 mm, Fig. 2E) and C-5941-1 × C-5941-5 (42 ± 7 mm, Fig. 2B).
Fig. 2.
Fruiting body formation from intra-strain crossing of single ascospore strains of Cordyceps militaris after 50 days of growth in brown rice medium. A, C-5736-1 × C-5736-4; B, C-5941-1 × C-5941-5; C, C-6040-1 × C-6040-6; D, C-7992-2 × C-7992-4 and E, C-8549-2 × C-8549-4.
Selection of single ascospore strains through inter-strain crossing by hyphal anastomosis
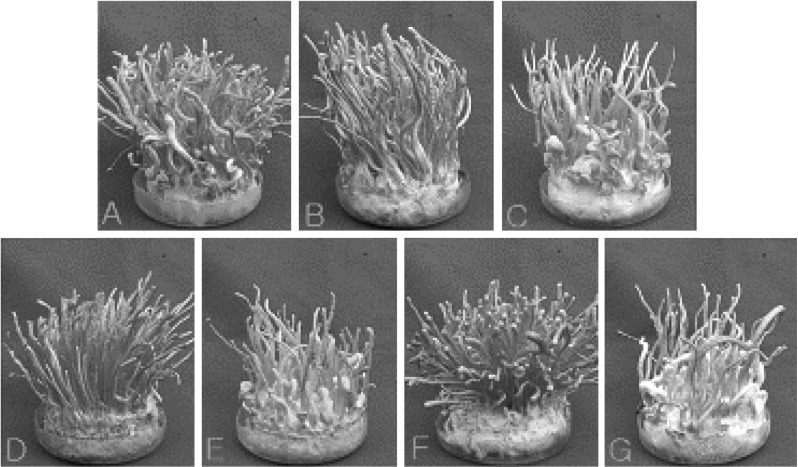
Among different inter-strain crossing, seven combinations produced profuse fruiting bodies in rice pupae medium (Table 9). The combination C-5736-1 × C-6040-1 produced the highest dry wt. (8.14 ± 0.10 g) of fruiting bodies (Fig. 3A), followed by C-5736-1 × C-7992-4 with dry wt. of 7.70 ± 0.14 g (Fig. 3B), C-6040-1 × C-7992-2 with dry wt. of 6.45 ± 0.13 g (Fig. 3F), C-5736-4 × C-7992-2 with dry wt. of 6.4 ± 0.15 g (Fig. 3D), C-5736-1 × C-8549-2 with dry wt. of 5.93 ± 0.11 g (Fig. 3C), C-6040-6 × C-7992-4 with dry wt. of 3.41 ± 0.18 g (Fig. 3G) and C-5941-5 × C-6040-1 with dry wt. of 2.94 ± 0.12 g (Fig. 3E). Fruiting bodies were mostly orange in color, but white stromata were also formed in few cases. Although fruiting body production from sister-crossing was generally found better than inter-strain crossing, but from dry wt. point of view, fruiting body production from out-crossing was similar to intra-strain crossing (Figs. 2, 3). Hence, for the selection of superior isolates, both intra-strain crossing and inter-strain crossing should be used simultaneously. Among inter-strain crossings also, the length of stromata varied considerably. The longest stromata of 79 ± 6 mm was produced by the cross C-5736-1 × C-7992-4 (Fig. 3B), followed by 78 ± 3 mm by C-5736-1 × C-6040-1 (Fig. 3A), 75 ± 7 mm by C-5736-4 × C-7992-2 (Fig. 3D), 71 ± 5 mm by C-6040-1 × C-7992-2 (Fig. 3F), 66 ± 4 mm by C-5736-1 × C-8549-2 (Fig. 3C), 55 ± 5 mm by C-6040-6 × C-7992-4 (Fig. 3G) and 52 ± 4 by C-5941-5 × C-6040-1 (Fig. 3E).
Fig. 3.
Fruiting body formation from inter-strain crossing of single ascospore strains of Cordyceps militaris after 50 days of growth in brown rice medium. A, C-5736-1 × C-6040-1; B, C-5736-1 × C-7992-4; C, C-5736-1 × C-8549-2; D, C-5736-4 × C-7992-2; E, C-5941-5 × C-6040-1; F, C-6040-1 × C-7992-2 and G, C-6040-6 × C-7992-4.
In addition to the cultural studies, molecular studies also have recently showed heterothallism in different Cordyceps species (Yokoyama et al., 2003, 2004). Very recently, heterothallism has been confirmed in C. takaomontana by both cultural and molecular studies (Yokoyama et al., 2005). To develop C. militaris as an important component of oriental medicine as well as modern medicine, it is very necessary to understand genetic and environmental factors to produce high potential fruiting bodies.
Acknowledgement
The authors wish to acknowledge the financial support from Korea Science and Engineering Foundation (KOSEF) to Entomopathogenic Fungal culture Collection (EFCC), Kangwon National University, Biogreen 21 Rural Development Administration and also wish to acknowledge to provide Cordyceps Research Institute for research facilities to carry out this study.
References
- 1.Choi IY, Choi JS, Lee WH, Yu YJ, Joung GT, Ju IO, Choi YK. The condition of production of artificial fruiting body of Cordyceps militaris. Korean J Mycol. 1999;27:243–248. [Google Scholar]
- 2.Harada Y, Akiyama N, Yamamoto K, Shirota Y. Production of Cordyceps militaris fruit body on artificially inoculated pupae of Mamestra brassicae in the laboratory. Trans Mycol Soc Japan. 1995;36:67–72. [Google Scholar]
- 3.Kim HO, Yun JW. A comparative study on the production of exopolysaccharides between two entomopathogenic fungi Cordyceps militaris and Cordyceps sinensis in submerged mycelial cultures. J Appl Microbiol. 2005;99:728–738. doi: 10.1111/j.1365-2672.2005.02682.x. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Yang S, Yang X, Chen Z, Li J. Anticarcinogenic effect and hormonal effect of Cordyceps militaris Link. Zhongguo Zhong Yao Za Zhi. 1997;22:111–113. [PubMed] [Google Scholar]
- 5.Mao XB, Zhong JJ. Significant effect of NH4+ on cordycepin production by submerged cultivation of medicinal mushroom Cordyceps militaris. Enzyme Microb Technol. 2006;38:343–350. [Google Scholar]
- 6.Sato H, Shimazu M. Stromata production for Cordyceps militaris (Clavicipitales: Clavicipitaceae) by injection of hyphal bodies to alternative host insects. Appl Entomol Zool. 2002;37:85–92. [Google Scholar]
- 7.Shrestha B. Growth characteristics of somatic mycelium and mating system of Cordyceps militaris (L. ex. Fr.) Link. Chuncheon, Republic of Korea: Kangwon National University; 2003. Ph.D. Dissertation. [Google Scholar]
- 8.Shrestha B, Choi SK, Kim HK, Kim TW, Sung JM. Genetic analysis of pigmentation in Cordyceps militaris. Mycobiology. 2005a;33:125–130. doi: 10.4489/MYCO.2005.33.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shrestha B, Han SK, Lee WH, Choi SK, Sung JM. Distribution and in vitro fruiting of Cordyceps militaris in Korea. Mycobiology. 2005b;33:178–181. doi: 10.4489/MYCO.2005.33.4.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrestha B, Kim HK, Sung GH, Spatafora JW, Sung JM. Bipolar heterothallism, a principal mating system of Cordyceps militaris in vitro. Biotech Bioprocess Engin. 2004a;9:440–446. [Google Scholar]
- 11.Shrestha B, Park YJ, Han SK, Choi SK, Sung JM. Instability in in vitro fruiting of Cordyceps militaris. J Mush Sc Prod. 2004b;2:140–144. [Google Scholar]
- 12.Sung JM. Insect-Borne Fungi of Korea. Seoul: Kyo-Hak Publishing Co. Ltd.; 1996. [Google Scholar]
- 13.Sung JM, Choi YS, Lee HK, Kim SH, Kim YO, Sung GH. Production of fruiting body using cultures of entomopathogenic fungal species. Korean J Mycol. 1999;27:15–19. [Google Scholar]
- 14.Sung JM, Choi YS, Shrestha B, Park YJ. Investigation on artificial fruiting of Cordyceps militaris. Korean J Mycol. 2002;30:6–10. [Google Scholar]
- 15.Won SY, Park EH. Anti-inflammatory and related pharmacological activities of cultured mycelial and fruiting bodies of Cordyceps militaris. J Ethnopharmacol. 2005;96:555–561. doi: 10.1016/j.jep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama E, Yamagishi K, Hara A. Structures of the mating-type loci of Cordyceps takaomontana. Appl Environ Microbiol. 2003;69:5019–5022. doi: 10.1128/AEM.69.8.5019-5022.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yokoyama E, Yamagishi K, Hara A. Development of a PCR-based mating type assay for Clavicipitaceae. FEMS Microbiol Lett. 2004;237:205–212. doi: 10.1016/j.femsle.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama E, Yamagishi K, Hara A. Heterothallism in Cordyceps takaomontana. FEMS Microbiol Lett. 2005;250:145–150. doi: 10.1016/j.femsle.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Yoo HS, Shin JW, Cho JH, Son CG, Lee YW, Park SY, Cho CK. Effects of Cordyceps militaris extract on angiogenesis and tumor growth. Acta Pharmacol Sin. 2004;25:657–665. [PubMed] [Google Scholar]
- 20.Zhao-Long W, Xiao XW, Wei YC. Inhibitory effects of Cordyceps sinensis and Cordyceps militaris on human glomerular mesangial cell proliferation induced by native LDL. Cell Biochem Funct. 2000;18:93–97. doi: 10.1002/(SICI)1099-0844(200006)18:2<93::AID-CBF854>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]