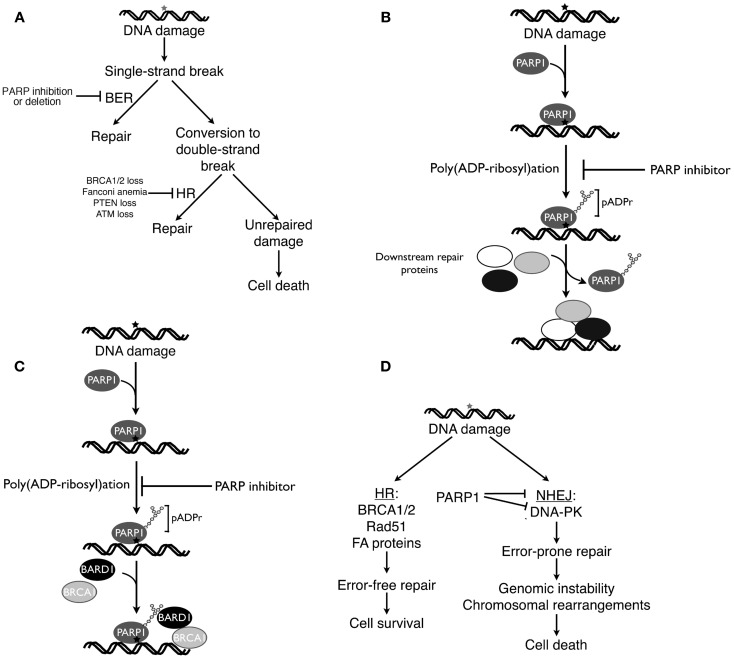
Figure 2.
Four current models of PARP inhibitor-induced cancer cell killing. (A), classical explanation of PARP inhibitor cytotoxicity in HR-deficient cells (124, 125). As described in the text, endogenous DNA damage is thought to result in DNA single-strand breaks, which ordinarily would be repaired by base excision repair (BER). If PARP inhibitors prevent BER, then persistent single-strand breaks are thought to be converted to DNA double-strand breaks, which would be repaired by HR in HR-proficient cells but remain unrepaired in HR-deficient cells. (B) Model emphasizing trapping of inhibited PARP1 at sites of DNA damage. According to this model, PARP1 binds to damaged DNA, synthesizes polymer, and then is released from the DNA so that repair enzymes can bind (51). Building on these observations, this model postulates that PARP inhibition results in failure of PARP1 to dissociate from sites of damage, leading to diminished access of other repair proteins, inhibited repair, and cell death. (C) Model emphasizing impaired recruitment of mutated BRCA1 in the presence of PARP inhibitors. As described by Li and Yu (134), recruitment of BRCA1 to DNA double-strand breaks requires both rapid binding of the BRCA1 binding partner BARD1 to pADPr and subsequent binding of a BRCA1-containing complex to phosphorylated H2AX at the break. Mutations that impair recruitment of the BRCA1-containing complex to phosphorylated H2AX render BRCA1 localization to sites of damage more dependent on the BARD1-pADPr interaction and, therefore, more sensitive to PARP inhibitors. (D), model emphasizing the role of activated NHEJ in PARP inhibitor killing. When DNA double-strand breaks occur, HR preferentially repairs them. In HR-deficient cells, however, double-strand breaks are more frequently repaired by the error-prone NHEJ pathway, resulting in mutations, chromosomal rearrangements, and NHEJ-mediated cell death. PARP inhibitors accelerate this process by removing a brake on NHEJ (116). (A,D) are modified from Patel et al. (116).