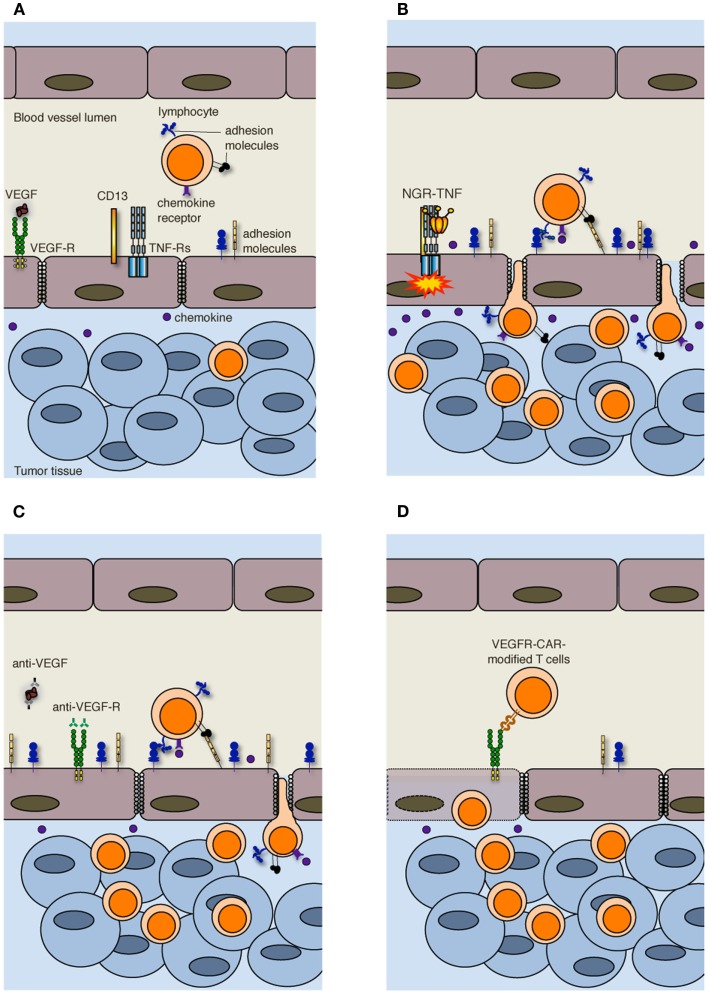
Figure 2.
Strategies to increase T cell infiltration into tumors. (A) Increased interstitial pressure, heterogeneous permeability and irregular blood flow, together with reduced expression of adhesion molecules on ECs, limit lymphocyte penetration in tumors. (B) NGR-TNF, which selectively binds CD13 expressed in ECs of neo-angiogenic vessels and favors the interaction of TNF with TNF receptors (TNF-Rs), alters tumor vessel permeability by loosening VE-cadherin dependent adherence junctions, induces up-regulation of adhesion molecules in ECs, and elicits the release of pro-inflammatory cytokines and chemokines, thereby favoring the recruitment and extravasation of T lymphocytes. (C) Anti-VEGF and anti-VEGF-R antibodies both transiently normalize the tumor-vasculature and overcome EC anergy, thus favoring T cell trafficking within tumors. (D) Also immunization against VEGF-R2 or the adoptive transfer of autologous T cells genetically engineered to express chimeric antigen receptor targeted against VEGF-R2 (VEGFR-CAR) favor tumor infiltration by T cells, although the mechanism has not yet been clarified. It has been proposed that VEGF-R-specific T cells kill both ECs and MDSCs and Tregs (not shown) that express VEGF-R.