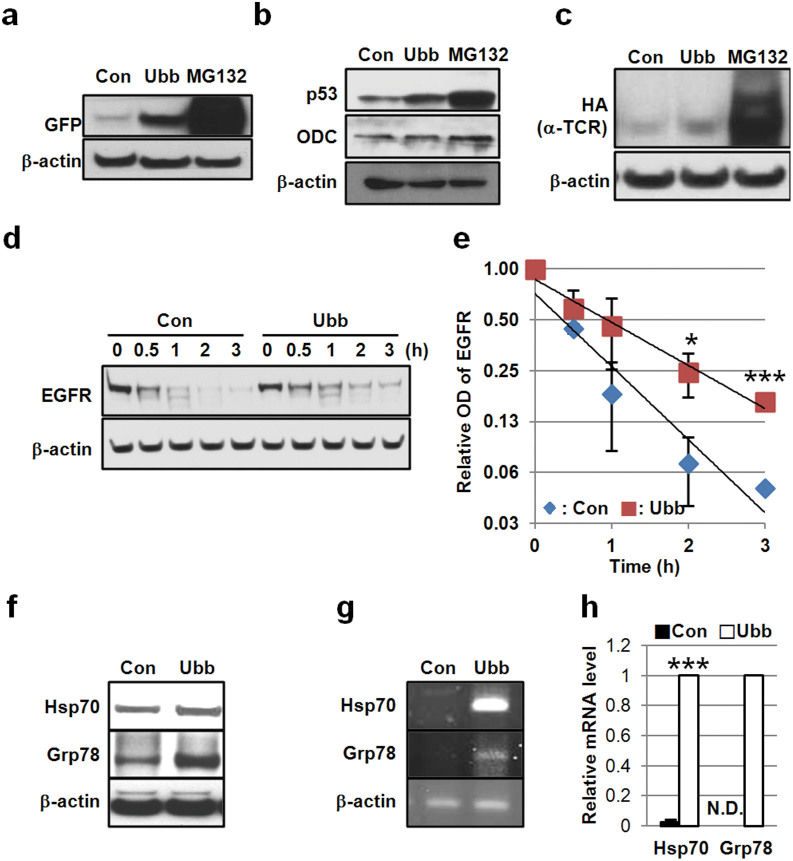
Figure 5. Ubb-KD stabilizes ubiquitination-dependent substrates and induces the expression of stress proteins.
(a) SH-SY5Y cells stably expressing GFPu were transfected with 20 nM control or Ubb siRNA for 48 h and then the level of GFPu was assessed by Western blot using anti-GFP antibody. β-actin was used as a control. (b) The levels of p53, ODC, and β-actin were compared in HepG2 cells transfected with 20 nM control or Ubb siRNA for 48 h. (c) HeLa cells stably expressing αTCR were transfected with 20 nM control or Ubb siRNA for 72 h, and the level of αTCR was compared by Western blotting with an anti-HA antibody. MG132 (10 μM) was treated for 24 h as positive control in (a), (b), and (c). (d) HeLa cells were transfected with control siRNA or Ubb siRNA (20 nM) for 48 h and then treated with EGF (100 ng/ml). The cells were harvested at the indicated times (0 h, 0.5 h, 1 h, 2 h, and 3 h), and the level of EGFR was compared by Western blot. (e) Graph showing optical densities of EGFR from the immunoblot in (d). Values were normalized by β-actin. (f) HeLa cells were transfected with control siRNA or Ubb siRNA (20 nM) for 72 h, and the levels of HSP70, GRP78, and β-actin were compared by Western blot. (g and h) Total RNA was isolated from siRNA-transfected HeLa cells after 48 h, and the levels of HSP70 and GRP78 mRNA were compared by PCR (g) and real-time PCR, values were normalized by β-actin mRNA (h). The data are shown as the mean ± SD (n = 2), *, p < 0.05; ***, p < 0.001; N.D., not detected.