Table 1.
Chrial γ-fluoroamines 10a–g via organocatalysis
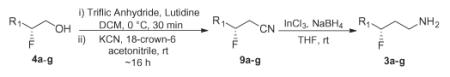
| Starting material | Yield of 9a–ga | Yield of 3a–gb | % eec |
|---|---|---|---|
| 4a | 9a, 71% | 3a, 87% | 94% |
| 4b | 9b, 92% | 3b, 84% | 87% |
| 4c | 9c, 73% | 3c, 86% | 92% |
| 4d | 9d, 93% | 3d, 83% | 96% |
| 4e | 9e, 77% | 3e, 90% | 96% |
| 4f | 9f, 82% | 3f, 89% | 95% |
| 4g | 9g, 84% | 3g, 73% | 96% |
All reactions were performed on a 1.0 mmol scale.
All reactions were performed on a 0.5 mmol scale.
Enantiomeric excess determined by 19F NMR using the (R)-Mosher amide of the final amine.
