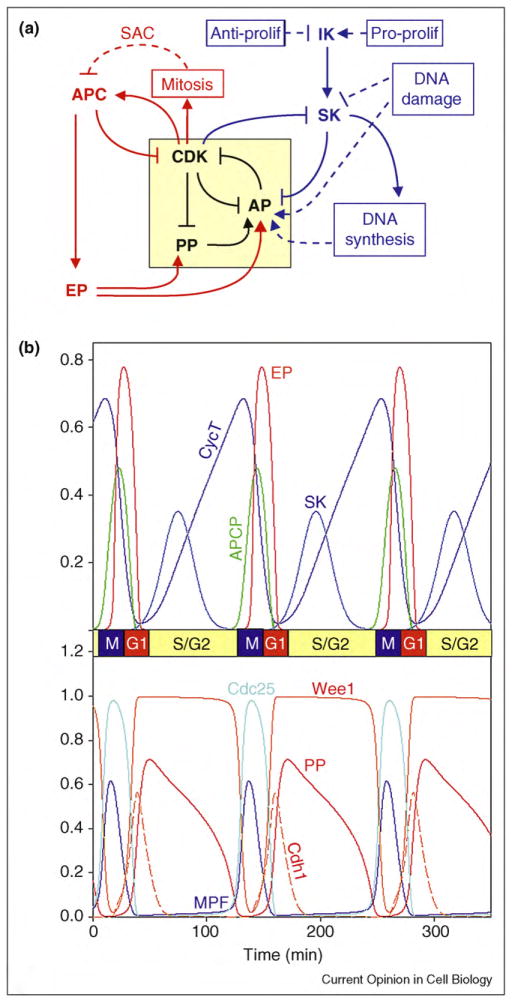
Figure 3.
General framework for cell cycle regulation by protein kinases and phosphatases. (a) Wiring diagram. The yellow box highlights the central toggle switch created by the ‘battle’ between CDK (cyclin B-dependent kinase) on one side and PP (protein phosphatase) and AP (antagonistic proteins) on the other side. In G1 phase, PP and AP are winning the battle, and CDK activity is low. The G1—S transition is promoted by an initiator kinase (IK) that upregulates a starter kinase SK. SK initiates DNA synthesis and downregulates AP. Rising activity of CDK drives the cell into mitosis and promotes mitotic-exit functions of the APC: degradation of cohesins, of cyclin B, and of an inhibitor of the exit phosphatase (EP). EP promotes return to G1 phase by activating PP and AP. Checkpoint signals are indicated by dashed lines. DNA synthesis delays progression into mitosis by activating an AP (Wee1). The spindle assembly checkpoint (SAC) delays exit from mitosis by inhibiting a component of APC (namely, Cdc20). Pro-proliferative and anti-proliferative signals determine whether a cell will start a new round of DNA replication and cell division by regulating the level of IK. DNA damage prevents DNA replication by inhibiting SK, and it prevents entry into mitosis by activating AP (Wee1 and CKI). (b) Numerical simulation. We convert scheme A into a set of nonlinear ordinary differential equations and compute an oscillatory solution for a reasonable choice of kinetic parameter values (for details, see Supplementary Material S4).