Figure 4.
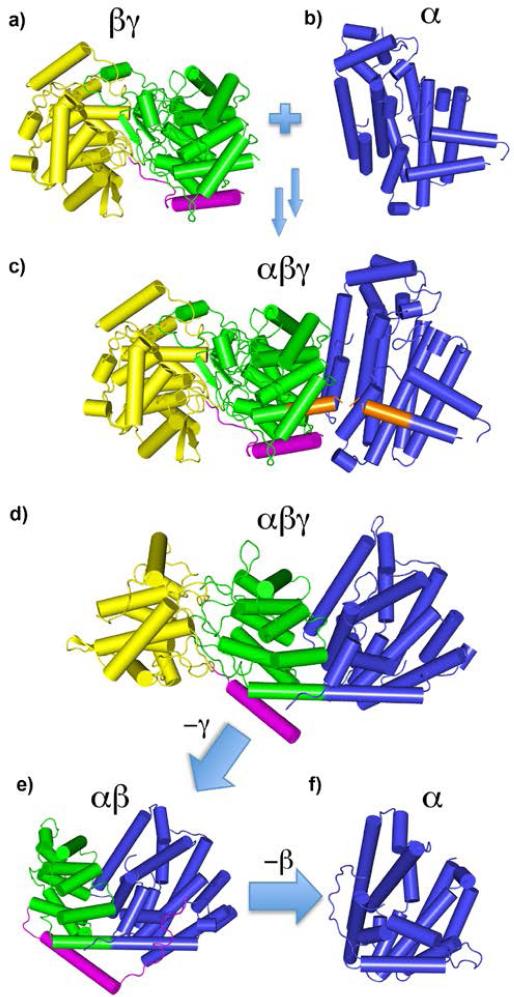
Genesis and evolution of terpene cyclases. a) Genes for ancestral βγ domain proteins (like SHC) fuse with genes for ancestral α-domain species like FPPS b) to generate αβγ three-helical domain diterpene cyclase, c) Orange shading indicates close proximity (~2.5 Å) of SHC C-terminus and FPPS N-terminus (from an α/αβ/βγ FPPS/EAS/SHC alignment). d) Structure of an actual diterpene cyclase, taxadiene synthase[51]. e) Loss of the γ domain yields an αβ protein, e.g. the sesquiterpene cyclase isoprene synthase. f) Further loss of the β domain yields other cyclases, e.g., pentalenene synthase, an α domain cyclase. Ancestral α and βγ-domain species presumably produced the FPP, GGPP and squalene used to produce lipids in Archaea; the derived families are much later arrivals. Note the N-terminal helix (magenta) portion is conserved in αβ, βγ, and αβγ proteins and is known to be required for activity.