Figure 6.
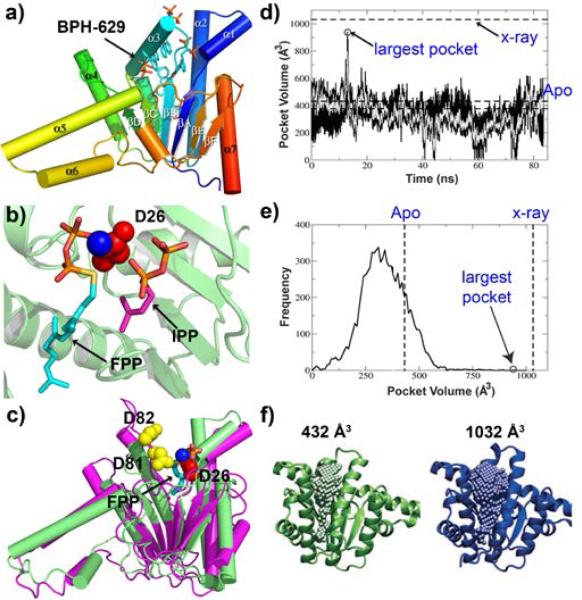
Structures and dynamics of the ζ prenyl transferase UPPS. a) Overall structure of a bisphosphonate-bound E. coli UPPS monomer. b) substrates (FPP and IPP) bound to UPPS active site. c) Structural alignment of the predicted structure of Rv3378c with UPPS. d) Molecular dynamics simulation of UPPS. Black, data taken every 10 ps; grey, every 100 ps. e) frequency of occurence of pocket versus pocket volume. The apo structure has a small pocket volume; the largest volume is close to that occupied by a large inhibitor.