Figure 4.
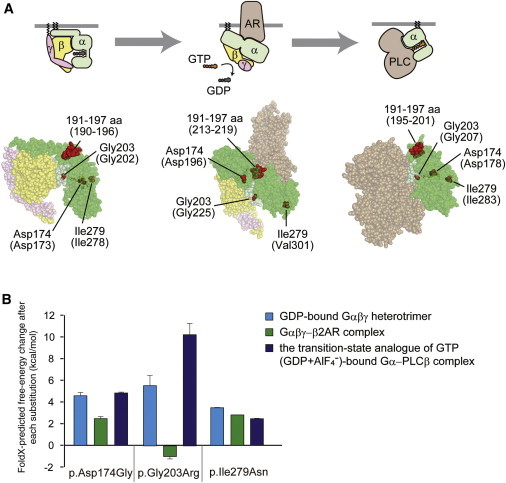
Structural Consideration of the Gα Amino Acid Substitutions in Some Complexed States
(A) Map of the amino acid substitution sites on the crystal structures of Gα-containing complexes: the GDP-bound inactive Gαiβγ heterotrimer (left), the nucleotide-free Gαsβγ in complex with an agonist-occupied monomeric β2AR (center), and the GDP+AlF4−-bound Gαq in complex with its effector PLCβ (right). Molecular structures are shown as space-filling representations (from PyMOL). Gα, Gβ, and Gγ subunits are colored green, yellow, and pink, respectively, and the switch I and switch II regions in the Gα subunit are in light green. The β2AR (center) and PLCβ (right) molecules are colored brown. The substituted sites are shown in red, and the indicated amino acid numbers correspond to human Gαo1 and, in parentheses, rat Gαi1 (UniProtKB/Swiss-Prot P10824) (left), bovine Gαs (UniProtKB/Swiss-Prot P04896) (center), and mouse Gαq (UniProtKB/Swiss-Prot P21279) (right). The illustrations above each model show the orientation of each subunit and the bound molecules.
(B) The free-energy change after each of the amino acid substitutions was estimated from calculations using FoldX software. Each error bar represents an average value with a SD.
