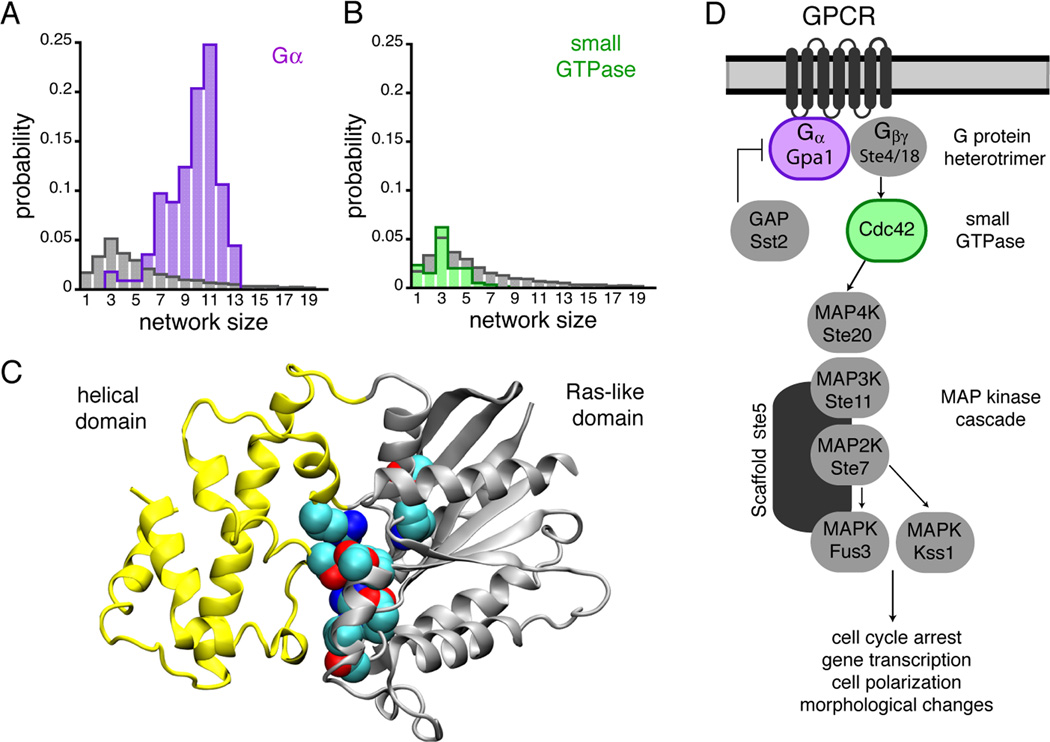
Figure 1.
Gα subunits contain structural motifs capable of proton sensing. (A, B) pHinder results showing that core networks of ionizable sidechains identified in 129 Gα structures (purple bars in A) were much larger and more prevalent than core networks identified in a reference set of 11,890 non-redundant PDB chains (gray bars in A and B) and 595 small GTPases (green bars in B). (C) Gα subunits consist of a helical (yellow) and Ras-like (gray) domain, whereas small GTPases consist of only a Ras-like domain. In Gα proteins the core pH-sensing network identified by pHinder and the sidechains responsible for Gα function reside at the interface of the helical and Ras-like domains. Collectively, the core networks observed in structures of mammalian Gαi consistently included residues Lys46, Asp150, Asp200, Asp229, Arg242, Lys270, and Lys277. (D) The core GPCR signaling components of the yeast pheromone pathway.