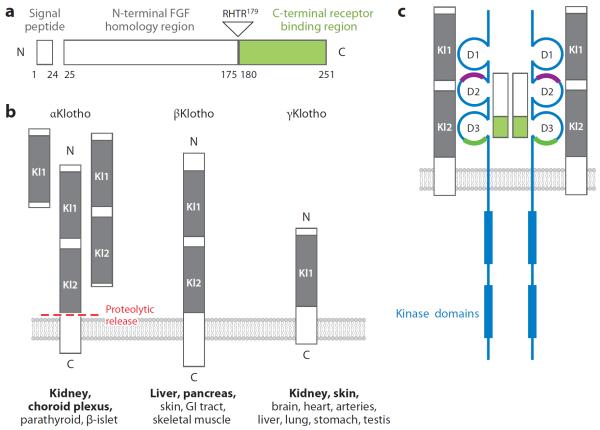
Figure 3.
Fibroblast growth factor (FGF) 23, Klotho family members, and the FGF23-FGF receptor (FGFR)-Klotho complex. (a) Structure of FGF23 showing the three main domains of this small polypeptide. The N terminus shares homology with other FGFs, whereas the C terminus is unique and binds to its cognate receptor (green). FGF23 circulates in the blood as both intact (full-length) and physiologically cleaved fragments. The C terminus binds but does not transactivate the FGFR-Klotho complex and can potentially function as a competitive antagonist. (b) Klotho family showing the three members identified to date in the mammalian genome, all of which are single-transmembrane proteins of varying lengths. Homologous motifs termed Kl domains are conserved. Soluble forms of αKlotho can be generated by alternative splicing of its transcript or by proteolytic cleavage of the transmembrane form by β-secretases into various body fluids. The major organs of expression of αKlotho, βKlotho, and γKlotho are shown in bold. (c) 2FGF23: 2FGFR:2αKlotho complex. The FGFR (blue) has three immunoglobulin-like domains (D1, D2, D3) that are stabilized by internal disulfide bridges. The heparin-binding region in the generic FGFR is shown (purple). For the endocrine FGF ligands, the coreceptor function of heparan sulfate is replaced by Klotho. αKlotho forms complexes with FGFR1c, FGFR3c, and FGFR4 and serves as the high-affinity receptor for FGF23. The ligand-binding region (green) interacts with the C terminus of FGF23. Klotho has a negligible intracellular region, whereas the FGFR has two kinase domains that sustain signal transduction. Abbreviations: GI, gastrointestinal; RHTR, arginine-histidine-threonine-arginine.