Abstract
Background: Ritalin has high tendency to be abused. It has been the main indication to control attention deficit hyperactivity disorder. The college students may seek for it to improve their memory, decrease the need for sleep (especially during exams), which at least partially, can be related to serotonergic system. Therefore, it seems worthy to evaluate the effect of Ritalin intake on mature brain. There are many studies on Ritalin effect on developing brain, but only few studies on adults are available. This study was undertaken to find Ritalin effect on serotonin transporter (SERT) density in medial frontal cortex (MFC) of mature rat. Methods: Thirty male Wistar rats were used in the study. Rats were assigned into five groups (n = 6 per group): one control, two Ritalin and two vehicle groups. Twelve rats received Ritalin (20 mg/kg/twice a day) orally for eleven continuous days. After one week of withdrawal and another two weeks of rest, in order to evaluate short-term effects of Ritalin, six rats were sacrificed. Another six rats were studied to detect the long-term effects of Ritalin; therefore, they were sacrificed 12 weeks after the previous group. The immunohistochemistry was performed to evaluate the results. Results: Immunohistochemistry studies showed a higher density of SERT in both 2 and 12 weeks after withdrawal from Ritalin intake in MFC of rat and there was no significant difference between these two groups. Conclusions: Our findings demonstrated both short- and long-term effects of Ritalin on frontal serotonergic system after withdrawal period.
Key Words: Ritalin, Serotonin, Rats
INTRODUCTION
Ritalin is a type II medication with a high tendency to be abused. It is mainly prescribed to control attention deficit hyperactivity disorder [1] and/or treatment of major depression [2]. It can improve memory [3] and attention [1], decrease the need for sleep and facilitate feeling of pleasure [4]. Also, it may induce alertness and sharpness [4]. Berridge et al. [5] showed that Ritalin could improve working memory after injection of small doses inside prefrontal cortex. These effects makes Ritalin to be abused among young adults, especially college students [1]. Previous studies have reported that tactile hallucinations following Ritalin intake are similar to marijuana sensory side-effects [6, 7]. However, the non-immediate consequences of Ritalin intake in adults have not been well understood yet [8].
It is believed that the known effects of Ritalin on nervous system is mediated partially through increase in synaptic concentration of dopamine via its re-uptake inhibition [9]. In addition, prior studies have reported effects of Ritalin on gene expression of rodent's brain similar to what happens in human brain after Ritalin intake [10] . Chronic Ritalin intake may result in permanent brain damage if prescribed in childhood [11].
Prefrontal cortex play the main role in highly integrated, executive, cognitive and behavioral functions such as non-verbal number processing [12]. Dorsal raphe nucleus is the main source of serotonergic ending of frontal cortex. Conversely, prefrontal cortex innervates the dorsal raphe nucleus. Prefrontal cortex may change serotonin release and serotonergic drive [13]. Serotonin is one of the crucial neurotransmitters in the frontal cortex [14] and can affect mood regulation [15], sleep cycle [15], memory formation [15], problem solving [13-15] and judgment [13-15]. Neocortex contains a high density of 5HT1A and 5HT2A receptors [13, 14]. There are different effects of serotonin on neocortical glutamatergic and gabaergic neurons. Serotonin through the 5HT2A receptors can increase glutamate release and results in excitatory effect [14], while by 5HT1A receptors, it activates gabaergic neurons and plays an inhibitory role [14]. Cortical integration by serotonin in layer 4 happens in medial frontal cortex (MFC) by five different serotonin receptors [13, 14].
The effect of Ritalin on cortical serotonin transmission has been studied before [16], but the consequences of chronic Ritalin intake on serotonergic system, especially in mature brain, are not clear [11, 16]. Some reports have shown the effects of other psychogenic agents such as Cannabis sativa (marijuana) on serotonin transporter expression in prefrontal cortex [8, 17]. To our knowledge, there are no or few studies about the impact of chronic Ritalin intake during adulthood [11, 18]. Therefore, in order to investigate short-term and long-term effects of chronic Ritalin intake in adults, this study was designed to evaluate the effect of chronic Ritalin intake on density of serotonin transporter (SERT) positive neurons in MFC of mature adult rat.
MATERIALS AND METHODS
Animals. All methods were conducted in accordance with laboratory animal care and approved by Ethical Committee of Iran University of Medical Sciences (Tehran, Iran). Male Wistar adult rats (n = 30, 250-300 g) were obtained from the Pasture Institute of Iran (Tehran). Animals, three in each cage, had free access to food and water and maintained at 21-24°C (room temperature) and 12 h light/darkness cycles.
Experimental design. There were five groups in our study, six animals in each: control, Ritalin + 12 WL (week latency), Ritalin + 2 WL, vehicle + 12 WL, vehicle + 2 WL. Ritalin + 12 WL group received Ritalin and were sacrificed after twelve weeks. Ritalin + 2 w group received Ritalin and were sacrificed after two weeks. Vehicle group received normal saline (0.5 ml) instead of Ritalin. There was no manipulation in control group. Ritalin (20 mg/kg) was gavaged twice a day for eleven consecutive days in treatment groups. Ritalin was obtained from Novartis (England). In order to study the short-term effects of chronic intake of Ritalin, transcardial brain fixation was carried out after 2 WL, while 12 WL were carried out in order to study the long-term effects.
Immunohistochemical analysis. Rats were anesthet-ized and perfused transcardially with 0.1 M PBS (pH 7.4), followed by 4% Phosphate-buffered paraformal-dehyde as fixative solution. The brains were removed and post fixed in the same fixative overnight. Then, the forebrains were cut and dehydrated in ascending alcohol series, cleared in xylene and infiltrated with paraffin after embedding in paraffin. The 5-μm coronal sections were serially collected from bregma 5.16 mm to 2.52 mm of forebrains with an interval of 30 μm between every two consecutive sections. All of the sections were processed for immunohistochemistry. The sections were incubated at 62ºC for 20 minutes, rehydrated in descending alcohols and immersed in 10% H2O2/methanol for 10 minutes to reduce endogenous peroxides activity. Then, they were washed in Tris buffer [H2NC (CH2OH)3, pH 7.4] and kept in citrate buffer (C6H5Na3O7.2H2O, pH 6) in autoclave to boil for 11 minutes. After cooling, the sections were washed in Tris wash buffer and incubated in BSA for 10 minutes. Afterward, they were incubated in the primary antibody (anti-SERT, Abcam, UK) with optimal dilution of 1:100 at 4°C overnight. The sections were washed again in Tris wash buffer (pH 7.4), then incubated in Envision Dual link System-horseradish peroxidase as secondary antibody (1:100, Dako, Denmark) for 1 hour. The sections were washed in Tris wash buffer (pH 7.4). To visualize the bound antibody, the sections were reacted with 3,3'-diaminobenzidine (Dako, Denmark) for 10 minutes, washed in Tris wash buffer (pH 7.4) and counterstained by immersing in hematoxylin for 10 minutes. Then they were washed in tap water for 3 minutes and dehydrated in ascending alcohols, cleared in xylene and covered with cover slip. Rat brain sections were used as positive based on company recommendation. For negative control, the sections were processed as described above except that the primary antibody was not used [19].
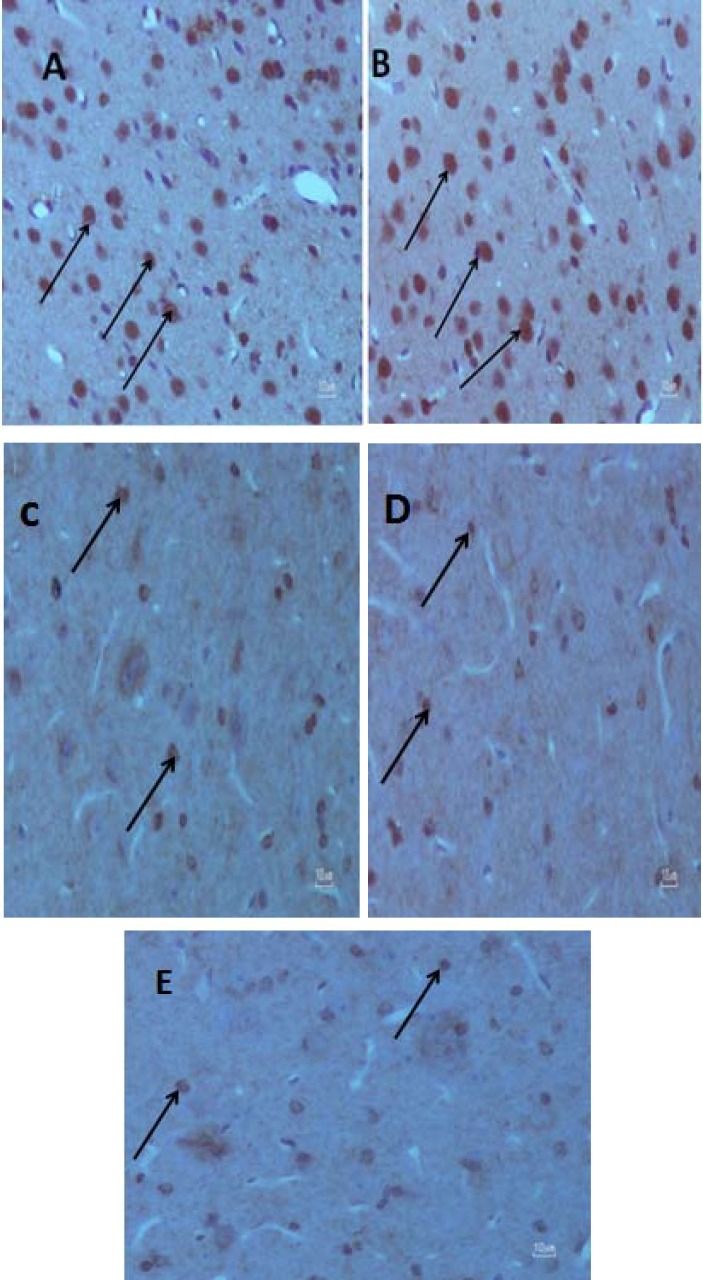
Measuring serotonin transporter density. Olympus AX70 microscopes (Japan) with a DP11 digital camera (magnification of 40×) were used to take the pictures. Five random fields of different MFC regions were investigated. OLYSIA BioReport software (Olympus optical Co. Ltd, Japan) was used. The number of SERT positive cells in the same five squares of a grid in each field was counted and the final count was reported as number per field (Fig. 1).
Fig. 1.
Effect of chronic Ritalin intake on rat medial frontal cortex. Images of the medial frontal cortex coronal sections in the (A) Ritalin 2 WL, (B) Ritalin 12 WL, (C) saline 2 WL, (D) saline 12 WL and E (control) (40 ×) groups. The arrows show SERT positive cells used to measure cell number in the experimental groups. The control and saline groups have less SERT positive cells (brown colored cells) compared to Ritalin-treated groups
Statistics. The data was analyzed by SPSS software using one-way analysis of variance (ANOVA) and Tukey's test as post test. Results were expressed as the mean ± SD and considered significant for P<0.05.
RESULTS
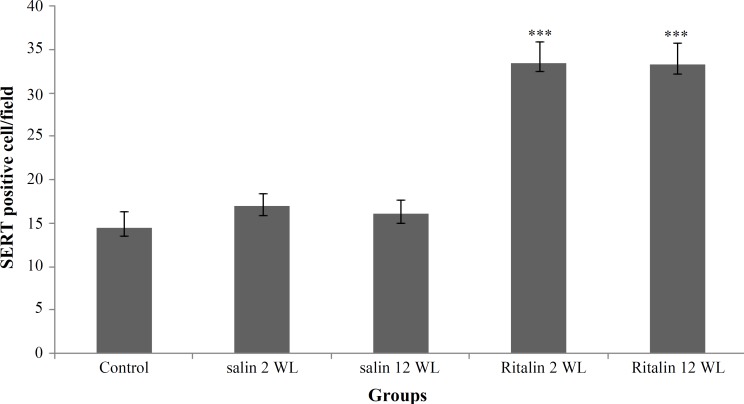
Density of SERT positive cells in MFC of five different groups were compared using one-way analysis of variance. Treatment with Ritalin or saline was considered among different groups (Table 1). Results of analysis showed the main effect of Ritalin intake on SERT positive cell numbers [F (4, 25) = 143.53, P<0.0001]. Tukey's post hoc analysis revealed differences between Ritalin-treated groups, with 2 and 12 WL after Ritalin withdrawal, compared to the control groups (P<0.0001). Mean of SERT positive cells in Ritalin-treated group with 2 WL after withdrawal was 33.5 ± 2.4. Also, it was 33.3 ± 2.4 per field in the group with 12 WL after Ritalin withdrawal compared to 14.5 ± 1.8 per mm3 in control groups (Fig. 2).
Table 1.
Number of serotonin transporter (SERT) positive cells in medial frontal cortex of rats treated with Ritalin or saline for eleven days (2 or 12 weeks after withdrawal week latency [WL])
| Groups | SERT positive cell number per field (mean ± SE) |
|---|---|
| Control | 14.5 ± 1.8 |
| Saline 2 WL | 17.0 ± 1.4 |
| Saline12 WL | 16.1 ± 1.5 |
| Ritalin 2 WL | 33.5 ± 2.4 |
| Ritalin12 WL | 33.3 ± 2.4 |
Fig. 2.
Number of serotonin transporter (SERT) positive cells in medial frontal cortex of rats treated with Ritalin or saline for eleven days. There was a significant increase in the SERT positive cell number (***P<0.0001), both long-term (12 WL) and short-term (2 WL) after withdrawal compared to control. Data are presented as mean ± SEM
Our results also showed no difference between densities of SERT positive cells in different Ritalin-treated groups with different withdrawal times.
DISCUSSION
Ritalin is believed to affect brain by dopamine modulation, but its role on serotonin load is controversy. There are documents about role of Ritalin just on brain dopaminergic/adrenergic systems [9, 11]; however, just very few documents insist on its role on serotonergic system [11, 16].
The main finding of this study was both long- and short-term increase of SERT density in MFC of male adult rat after chronic Ritalin intake. Some reports have confirmed the effect of Ritalin on brain SERT level [16, 20]. Findings indicate that children who take anti-asthmatic medication, which lowers serotonin level in brain and plasma, may be forced to take Ritalin in order to increase brain serotonin level [21, 22].
In 1999, Berger [23] found that hyperactivity in attention deficit hyperactivity disorder victims is due to serotonin shortage and Ritalin balances brain serotonin and dopamine. In addition, Ritalin does not affect brain directly by dopaminergic system, but serotonin system stimulates its receptors directly which mimics Ritalin [16, 23].
Changes in brain serotonin level may cause different biological and behavioral changes [14, 15]. SERT is the main factor to regulate brain serotonin concentration [14, 15]. SERT density may have profound effects on frontal cortex activity [13-15]. Serotonergic system is involved both in mood regulation and neuropathology of its disorders [15]. Specific mechanisms of actions of anti-depressants are mediated via modulation of serotonergic system [14]. Some anti-depressants such as SERT inhibitors may antagonize SERT and promote its internalization and/or increase SERT gene expression, which is referred as neuroadaptation [24].
It is important to find which factors may change serotonin concentration in brain. Raphe nucleus has an important role on synthesis and transmission of serotonin; therefore, any change in its activity can easily affect brain serotonin level [14]. Many other explanations for change in SERT density have been suggested in previous studies [24, 25]. Increased expression of SERT gene is possibly because of accelerated serotonin degradation, for example in response to activation of serotonergic system function induced by social stress [25]. Correlation between expressions of the two main molecules (monoamino-oxidize A and SERT) involved in clearance of synaptic serotonin has also been reported. Significant positive correlation between monoamine oxidase A and SERT mRNA levels suggests common pathways in regulation of these genes transcriptional activity [25].
But how Amphetamine-like medications modulate brain serotonin level? The exact mechanism of action of amphetamines on SERT expression has not been well understood yet [26]. SERT is a phosphoprotein by itself; phosphoprotein molecules are proteins attached to a substance containing phosphoric acid [27]. In this process, SERT activity can be modulated by kinase/phosphatase enzymes [27, 28]. SERT protein can be phosphorylated by various kinases.
Phosphorylated SERT will turn inside the cell and gets inactivated. Also, phosphatase dephosphorylate SERT proteins and expose them to cell surface [27, 28]. Therefore, phosphorylated SERT is inactive and moves inside the cell, while dephosphorylated SERT is exposed and located outside [27, 28]. Any factor with such ability can modulate SERT concentration. Some studies have shown a complex correlation between phospho kinase C and SERT regulation [27, 28]. Effect of phospho kinase C on SERT is direct and quick; it internalizes SERT rapidly. On the other hand, effects of other protein kinases such as phospho kinase A and phospho kinase G on SERT have not been well understood yet [27, 29]. PKG activity and its interaction with nitric oxide signaling has also been reported to be effective in modifying SERT expression following adenosine receptors activation [29]. In addition, Ritalin mechanism of action on dopamine is modulated by dopamine active transporter phosphorylation/deposphorylation [30].
It could be assumed that Ritalin possibly affects SERT by phosphorylation of psychostimulant-sensitive neurotransmitter transporters. This mechanism is counted as one of the most possible regulatory system of these proteins [31].
Our next finding was the duration of Ritalin patent on brain. We found both short- and long-term SERT density increase after Ritalin chronic intake .It has been shown that behavioral effects of psychostimulants may remain long term after initial exposure. For example, Ritalin role as an anti-depressant, especially in case of resistant major depression [2], may have both short- and long-term effects. Especial psychostimulants are reported to affect adult brain, even for months in mammals and human [32]. An example of long-term effects of psychostimulants is behavioral sensitization. In sensitization phenomenon, repeated exposure to psychostimulants (including methylphenidate) results in locomotive hypersensitivity even months after the last intake [32, 33], which may extend long time after psychostimulant administration [32].
Warren et al. [28] showed that cocaine can result in two different neuropsychological conditions: mood changes (possibly reversible) and cognitive disorders.
The long-term effect of Ritalin on brain serotonin pool seems to be long lasting and related to gene regulation system changes. We also found short-term effect of Ritalin on rat MFC.
Polymorphism of SERT gene could be responsible for short-term behavioral changes in children taking Ritalin [31]; however, it may happen in adult brain.
Nevertheless, it is not clear that changes in SERT expression following Ritalin intake is related to these behavioral effects or not. Therefore, this issue needs further investigations. Different documents have shown that serotonin (serotonin-specific reuptake inhibitor medication) potentiate Ritalin effect [34, 35].
This finding show that concomitant SSRI treatment with Ritalin exacerbates the expression of two different transcription factors: a) early growth response protein 1, also known as zinc finger protein 225 or nerve growth factor-induced protein A and b) C-Fos in sensoriomotor striatum [34].
Other findings show that in addition to magnifying of Ritalin specific gene regulation by SSRI, serotonin by itself can facilitate dopamine-induced gene regulation [32, 34]. Therefore, serotonin may also indirectly potentiate the effects of Ritalin on brain.
In conclusion, chronic use of Ritalin affects brain serotonergic system modulation by SERT density increase, which is seen in a short- and long-term period of time. In this study, we observed an increase in SERT positive neurons in MFC, which is visible after chronic Ritalin intake.
References
- 1.Mannuzza S, Klein RG, Truong NL, Moulton JL 3rd, Roizen ER, Howell KH, et al. Age of methylphenidate treatment initiation in children with ADHD and later substance abuse: prospective follow-up into adulthood. Am J Psychiatry. 2008 May;165(5):604–9. doi: 10.1176/appi.ajp.2008.07091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobson A. The use of Ritalin in psychotherapy of depressions of the aged. Psychiatr Q. 1958 Jul;32(3):474–83. doi: 10.1007/BF01563517. [DOI] [PubMed] [Google Scholar]
- 3.Mehta MA, Owen AM, Sahakian BJ, Mavaddat N, Pickard JD, Robbins TW. Methylphenidate enhances working memory by modulating discrete frontal and parietal lobe regions in the human brain. J Neurosci. 2000 Mar;20(6):RC65. doi: 10.1523/JNEUROSCI.20-06-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keane H. Pleasure and discipline in the uses of Ritalin. Int J Drug Policy. 2008 Oct;19(5):401–9. doi: 10.1016/j.drugpo.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, et al. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006 Nov;60(10):1111–20. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Brust JC. Abused agents: acute effects, withdrawal, and treatment. Continuum. 2004 Oct;10(5):14–47. [Google Scholar]
- 7.Devlin RJ and Henry JA. Clinical review: Major consequences of illicit drug consumption. Crit Care. 2008;12(1) doi: 10.1186/cc6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tunving K. Psychiatric effects of cannabis use. Acta Psychiatr Scand. 1985 Sep;72(3):209–17. doi: 10.1111/j.1600-0447.1985.tb02597.x. [DOI] [PubMed] [Google Scholar]
- 9.Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001 Jan;21(2):RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adriani W, Leo D, Greco D, Rea M, di Porzio U, Laviola G, et al. Methylphenidate administration to adolescent rats determines plastic changes on reward-related behavior and striatal gene expression. Neuropsychopharmacology. 2005 Sep;31(9):1946–56. doi: 10.1038/sj.npp.1300962. [DOI] [PubMed] [Google Scholar]
- 11.Gray JD, Punsoni M, Tabori NE, Melton JT, Fanslow V, Ward MJ, et al. Methylphenidate administration to juvenile rats alters brain areas involved in cognition, motivated behaviors, appetite, and stress. J Neurosci. 2007 Jul;27(27):7196–207. doi: 10.1523/JNEUROSCI.0109-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spencer RC, Klein RM, Berridge CW. Psychostimulants act within the prefrontal cortex to improve cognitive function. Biol Psychiatry. 2012 Aug;72(3):221–7. doi: 10.1016/j.biopsych.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Bockstaele EJ, Biswas A, Pickel VM. Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res. 1993 Oct;624(1-2):188–98. doi: 10.1016/0006-8993(93)90077-z. [DOI] [PubMed] [Google Scholar]
- 14.Murphy DL, Andrews AM, Wichems CH, Li Q, Tohda M, Greenberg B. Brain serotonin neurotransmission: an overview and update with an emphasis on serotonin subsystem heterogeneity, multiple receptors, interactions with other neurotransmitter systems, and consequent implications for understanding the actions of serotonergic drugs. J Clin Psychiatry. 1998;59(Suppl 15):4–12. [PubMed] [Google Scholar]
- 15.Muller CP, Jacobs B. Handbook of Behavioral Neurobiology of Serotonin. Academic Press; 2009. [Google Scholar]
- 16.Volkow ND, Gatley SJ, Fowler JS, Wang GJ, Swanson J. Serotonin and the therapeutic effects of ritalin. Science. 2000 Apr;288(5463) doi: 10.1126/science.288.5463.11a. [DOI] [PubMed] [Google Scholar]
- 17.McCann UD, Lowe KA, Ricaurte GA. Review: Long-lasting effects of recreational drugs of abuse on the central nervous system. Neuroscientist. 1997 Nov;3(6):399–411. [Google Scholar]
- 18.Barron E, Yang PB, Swann AC, Dafny N. Adolescent and adult male spontaneous hyperactive rats (SHR) respond differently to acute and chronic methylphenidate (Ritalin) Int J Neurosci. 2009;119(1):40–58. doi: 10.1080/00207450802330546. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto H, Fujimiya M, Shirai Y, Nakashita M, Oyasu M, Saito N. Immunohistochemical localization of serotonin transporter in normal and colchicine treated rat brain. Neurosci Res. 1998 Dec;32(4):305–12. doi: 10.1016/s0168-0102(98)00097-2. [DOI] [PubMed] [Google Scholar]
- 20.Gatley SJ, Pan D, Chen R, Chaturvedi G, Ding YS. Affinities of methylphenidate derivatives for dopamine, norepinephrine and serotonin transporters. Life Sci. 1996;58(12):231–9. doi: 10.1016/0024-3205(96)00052-5. [DOI] [PubMed] [Google Scholar]
- 21.McQuaid EL, Kopel SJ, Nassau JH, Nassau Behavioral adjustment in children with asthma: a meta-analysis. J Dev Behav Pediatr. 2001 Dec;22(6):430–9. doi: 10.1097/00004703-200112000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Pretorius E. Asthma medication may influence the psychological functioning of children. Med hypotheses. 2004;63(3):409–13. doi: 10.1016/j.mehy.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 23.Berger A. Ritalin may influence serotonin balance in hyperactive children. BMJ. 1999 Jan;318(7178) doi: 10.1136/bmj.318.7178.212b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benmansour S, Cecchi M, Morilak DA, Gerhardt GA, Javors MA, Gould GG, et al. Effects of chronic antidepressant treatments on serotonin transporter function, density, and mRNA level. J Neurosci. 1999 Dec;19(23):10494–501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Filipenko ML, Beilina AG, Alekseyenko OV, Dolgov VV, Kudryavtseva NN. Repeated experience of social defeats increases serotonin transporter and monoamine oxidase A mRNA levels in raphe nuclei of male mice. Neurosci Lett. 2002 Mar;321(1-2):25–8. doi: 10.1016/s0304-3940(01)02495-8. [DOI] [PubMed] [Google Scholar]
- 26.Elliott JM, Beveridge TJ. Psychostimulants and monoamine transporters: upsetting the balance. Curr Opin Pharmacol. 2005 Feb;5(1):94–100. doi: 10.1016/j.coph.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Ramamoorthy S, Blakely RD. Phosphorylation and sequestration of serotonin transporters differentially modulated by psychostimulants. Science. 1999 Jul;285(5428):763–6. doi: 10.1126/science.285.5428.763. [DOI] [PubMed] [Google Scholar]
- 28.Jayanthi LD, Samuvel DJ, Blakely RD, Ramamoorthy S. Evidence for biphasic effects of protein kinase C on serotonin transporter function, endocytosis, and phosphorylation. Mol Pharmacol. 2005 Jun;67(6):2077–2087. doi: 10.1124/mol.104.009555. [DOI] [PubMed] [Google Scholar]
- 29.Zhu CB, Hewlett WA, Feoktistov I, Biaggioni I, Blakely RD. Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol Pharmacol. 2004 Jun;65(6):1462–74. doi: 10.1124/mol.65.6.1462. [DOI] [PubMed] [Google Scholar]
- 30.Loder MK, Melikian HE. The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem. 2003 Jun;278(24):22168–74. doi: 10.1074/jbc.M301845200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thakur GA, Grizenko N, Sengupta SM, Schmitz N, Joober R. The 5-HTTLPR polymorphism of the serotonin transporter gene and short term behavioral response to methylphenidate in children with ADHD. BMC Psychiatry. 2010 Jun;10:50. doi: 10.1186/1471-244X-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricaurte GA, schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980 Jul;193(1):153–63. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- 33.Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, Garon MG. Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science. 1999 Jan;283(5400):397–401. doi: 10.1126/science.283.5400.397. [DOI] [PubMed] [Google Scholar]
- 34.Van Waes V, Beverley J, Marinelli M, Steiner H. Selective serotonin reuptake inhibitor antidepressants potentiate methylphenidate (Ritalin)‐induced gene regulation in the adolescent striatum. Eur J Neurosci. 2010 Aug;32(3):435–47. doi: 10.1111/j.1460-9568.2010.07294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Warren BL, lniguez SD, Alcantara LF, Wright KN, Parise EM, Weakley SK, et al. Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward-and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J Neurosci. 2011 Jul;31(28):10347–58. doi: 10.1523/JNEUROSCI.1470-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]