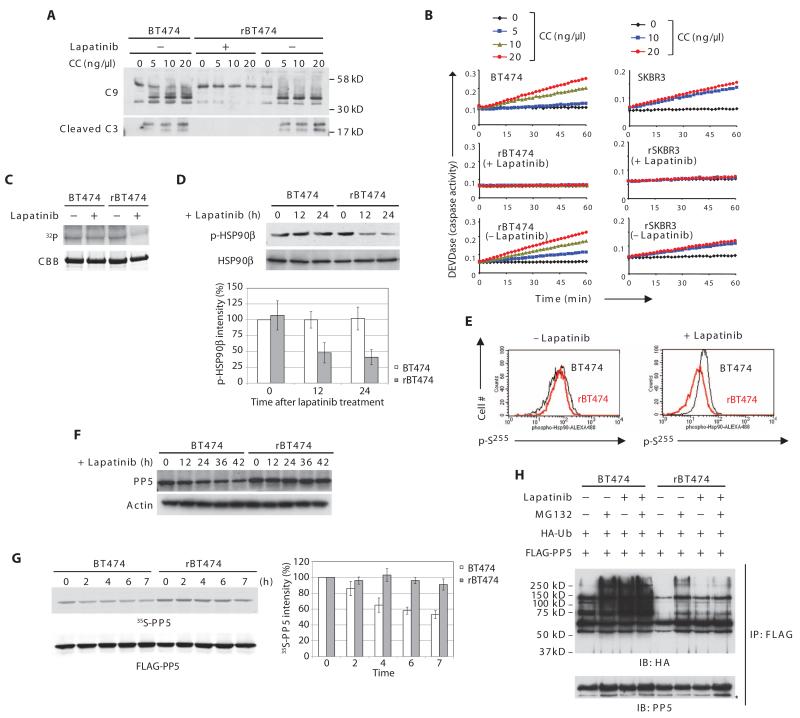
Fig. 2. PP5 stability and post-cytochrome c protection in lapatinib-resistant cells.
(A) rBT474 cells were cultured in the presence or absence of lapatinib for 1 week, whereas BT474 cells were maintained without lapatinib. Cytosolic lysates prepared from BT474 or rBT474 cells were incubated with 2′-deoxyadenosine 5′-triphosphate (dATP) and various amounts of cytochrome c (CC). Immunoblotting was performed for caspase-9 (cleaved and noncleaved) and cleaved caspase-3 (C9 and C3, respectively). n = 5 independent experiments. (B) Caspase-3 activity was assayed by measuring cleavage of DEVD-pNA after incubation of the cell lysates with dATP and various concentrations of cytochrome c. A representative result of five independent experiments is shown. (C) BT474 or rBT474 cells were cultured in the presence or absence of lapatinib for 6 hours and in the presence of z-VAD. Recombinant His-tagged HSP90β protein on nickel beads was incubated with the cell lysates in the presence of [γ-32P]ATP. HSP90β phosphorylation (32P) and total HSP90β protein [Coomassie brilliant blue (CBB)] are shown. n = 3 independent experiments. (D) BT474 or rBT474 cells treated with lapatinib for the indicated times were analyzed by immunoblotting for phospho-HSP90β (Ser226) and total HSP90β. Densitometric analysis of phospho-HSP90β (p-HSP90β) normalized to total HSP90β from four independent experiments is shown (means ± SEM). Phospho-HSP90β at the 0 time point in BT474 cells was set at 100%. (E) BT474 or rBT474 cells treated with lapatinib were fixed and incubated with phospho-Hsp90β (Ser255) antibody followed by an Alexa Fluor 488-conjugated secondary antibody. The phosphorylation status of Ser255 was analyzed by FACS (fluorescence-activated cell sorting; black: BT474; red: rBT474). n = 2 independent experiments. (F) BT474 and rBT474 treated with lapatinib for the indicated times were immunoblotted for PP5 and actin. n = 3 independent experiments. (G) BT474 or rBT474 cells expressing FLAG-PP5 were labeled by the addition of 35[S]Met and 35[S]Cys and cultured in the presence of lapatinib. 35S radioactivity (35S-labeled PP5) was measured in FLAG-PP5 immunoprecipitates collected at the indicated time points. Densitometric analysis of the abundance of 35S-labeled PP5 from five independent experiments is shown (means ± SEM). The 35S-labeled PP5 abundance at 0 time point was set at 100%. (H) BT474 or rBT474 cells expressing FLAG-PP5 and HA-Ub were cultured in the presence or absence of lapatinib and harvested after treatment with MG132. FLAG-PP5 immunoprecipitates were immunoblotted for HA to analyze PP5 ubiquitylation (*IgG heavy chain). n = 3 independent experiments.