Figure 1.
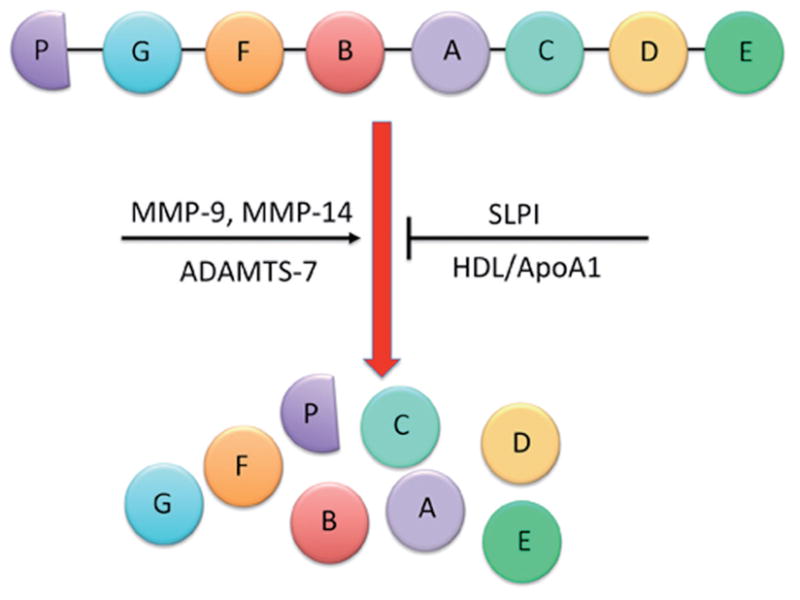
Proteolytic cleavage of PGRN. The human PGRN gene contains a number of granulin-like domains. After secretion, the full-length protein can be proteolytically cleaved between the granulin-like domains by metalloproteinases such as matrix metalloproteinase-9 (MMP-9), MMP-14, and ADAMTS-7. Complete cleavage of full-length PGRN results in granulin peptides (GRN A–G and paragranulin) that are approximately 6 kDa in size, though intermediary cleavage products have also been identified. The proteolytic processing of full-length PGRN can be inhibited when secretory leukocyte protease inhibitor (SLPI) or high-density lipoprotein/apolipoprotein A-1 (HDL/ApoA-1) binds to full-length PGRN to prevent its cleavage
