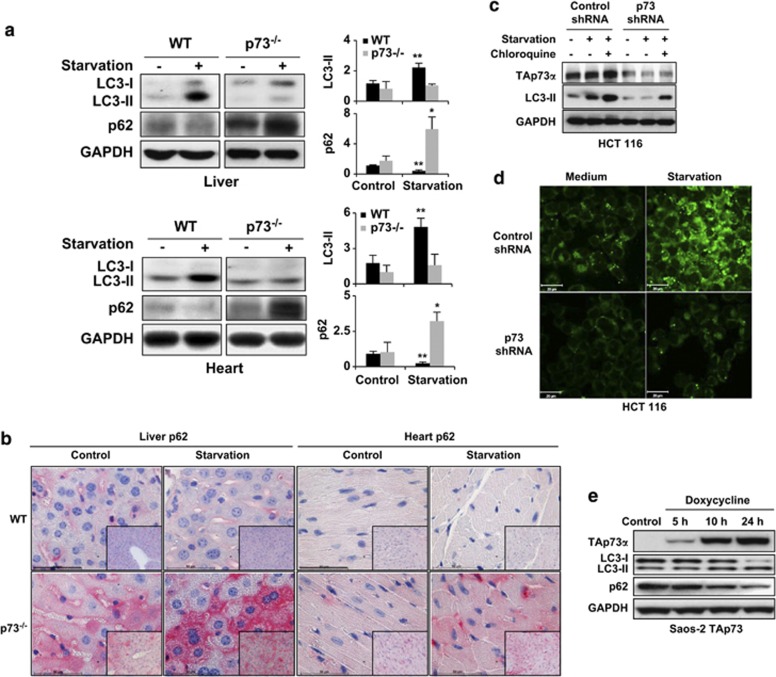
Figure 2.
p73 regulates autophagy both in vivo and in vitro. (a) Immunoblotting. Livers and hearts were taken from WT and p73-deficient mice under normal feeding conditions and after food deprivation for 24 h (starvation). p73-deficient mice are unable to increase LC3-II levels and accumulate p62 upon starvation. Results are representative of three independent experiments. (Right) The fold changes in expression levels, compared with WT control, are presented. *P<0.05; **P<0.01, n=3. (b) Immunohistochemistry. The same tissues as in panel a were stained with anti-p62 antibody. The p73-deficient mice show slightly increased p62 levels compared with WT mice under normal conditions and a strong p62 accumulation after starvation. Bar, 50 μm. Original magnifications, × 630 and × 200 (lower right corners). Results are representative of three independent experiments. (c) Immunoblotting. HCT 116 cells were infected with lentiviral control shRNA or p73 shRNA. 48 h after infection, cells were starved in an EBSS medium for 1 h. Control (unspecific) shRNA-treated cells had higher LC3-II levels than did p73 shRNA-treated cells. Moreover, whereas the control shRNA-treated cells increased their LC3-II levels upon starvation, p73 shRNA-treated cells exhibited no response. LC3-I was not detectable in this experiment. (d) Autodot staining. Significantly increased staining after starvation was seen in control but not in p73-deficient HCT 116 cells. Bar, 20 μm. Similar results were obtained in HaCaT cells, including using other methods for inducing autophagy (see Supplementary Figure S8). (e) Immunoblotting. Saos-2 cells inducible for TAp73α were treated with doxycycline (2.5 μg/ml) for the indicated time periods. Increased levels of TAp73α resulted in increased LC3-II and reduced p62 levels. Other p73 and p63 isoforms, as well as p53, were without effect in this system (see Supplementary Figure S9)