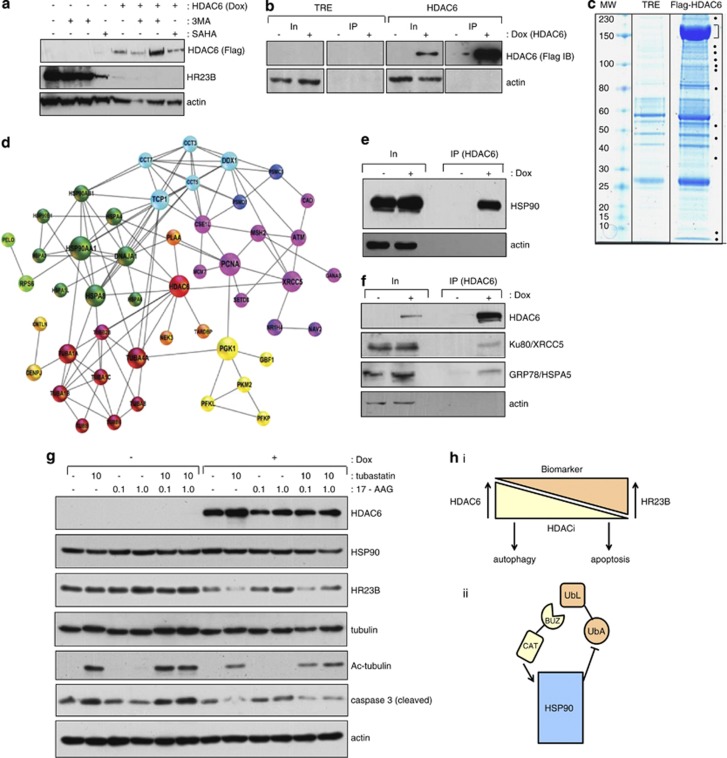
Figure 5.
Analysis of HDAC6 interacting proteins. (a) The stable Flag-HDAC6 cell line was treated with (+) or without (−) doxyclycline, and the autophagy inhibitor 3-MA (5 and 10 mM), or SAHA (10 μM) as indicated. (b) Both control cells expressing the vector only (TRE) and stable cells expressing Flag-HDAC6 were induced with doxycyclin (+ 1 μM) for 60 h or left uninduced. Extracts were immunoprecipitated with anti-Flag antibody and subsequently immunoblotted with anti-Flag antibody. Input levels are shown together with actin as loading control, and note under these conditions low level expression of HR23B (a). (c) Co-immunoprecipitation of the Flag-HDAC6 and the empty vector TRE control were stained with a colloidal blue. Dots indicate specific bands in the HDAC6 co-immunoprecipite which were excised and analysed by mass spectrometry. The prominent band around 150 kDa (indicated by the bracket) is the immuno-enriched Flag-HDAC6. Supplementary Table 1 provides further details of the interacting proteins. (d) In silico analysis of HDAC6 interacting proteins found by mass spectrometry are shown for which experimental data have been annotated in STRING.50 The proteins were clustered by the Markov Cluster Algorithm (MCL) and every colour corresponds to one protein cluster. The diameter of the circle represents the betweenness centrality (calculated by the programme Gephi) and is a measure of the importance of the protein in the analysed network. (e) The stable Flag-HDAC6 cell line was treated with (+) or without (−) doxycycline. Extracts were immunoprecipitated with anti-Flag followed by immunoblotting with anti-HSP90. (f) The stable Flag-HDAC6 cell line was treated with (+) or without (−) doxycycline. Extracts were immunoprecipitated with anti-Flag followed by immunoblotting with anti-Ku80/XRCC5 or anti-GRP78/HSPA5. (g) The immunoblot shows the endogenous levels of HR23B upon induction (Dox treatment) of the Flag-HDAC6 stable inducible cell line. Two days after the induction, the HDAC6 specific inhibitor tubastatin and/or (10 μM) the HSP90 specific inhibitor 17-AAG (0.1 or 1 μM) were added and the cells were harvested 2 days later. Acetylated tubulin provides a control for the tubastatin treatment. Extracts were immunoblotted with anti-HSP90, anti-HR23B, anti-tubulin, anti-acetylated tubulin, anti-caspase 3 (cleaved) and anti-actin, as indicated. (h)Model for interplay between HR23B and HDAC6 in regulating the cellular response to HDAC inhibitors. It is envisaged that the level of HR23B and HDAC6 has an impact on the response to HDAC inhibitors, reflected as apoptotic or autophagocytic outcomes of drug treatment (i). The mechanism which underpins HDAC6 and its effect on HR23B does not require catalytic activity but involves the interplay with the HSP90 (via HDAC6 BUZ domain interacting with HR23B UbL domain; (ii). Note that HDAC6 has an established role in activating HSP90 chaperone function through its catalytic (CAT) domain