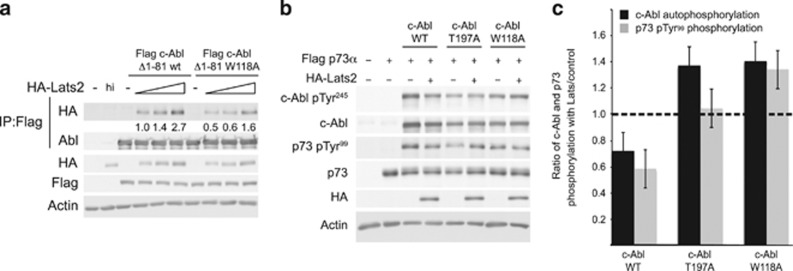
Figure 6.
c-Abl SH3 mutants are resistant to inhibition by Lats2. (a) Mutation of c-Abl at W118 impairs binding to Lats2. HEK293 cells were transfected with the constructs indicated, with 0.25, 0.5, and 1 μg of HA-Lats2 plasmids used. Flag-c-Abl constructs were immunoprecipitated and IP and total extracts were analyzed by immunoblotting. Quantification of the amount of HA-Lats2 that co-immunoprecipitated, normalized to the amount of immunoprecipitated c-Abl, is presented. (b,c) c-Abl T197A and W118A mutants are not inhibited by Lats2. HEK293 cells were transfected with plasmids expressing the proteins indicated. (b) Extracts were analyzed by immunoblotting. (c) The ratio of c-Abl autophosphorylation in the presence of Lats2 versus control (black bars), and the ratio of p73 pTyr99 in the presence of Lats2 versus control (gray bars) is presented. The level of c-Abl autophosphorylation was normalized to the amount of total c-Abl, and the level of phosphorylated p73 was normalized to total p73. Ratios were calculated from three independent experiments. The dashed line represents a ratio of 1, where phosphorylation of c-Abl and p73 is the same in the presence or absence of Lats2. Ratios less than 1 indicate inhibition of phosphorylation in the presence of Lats2