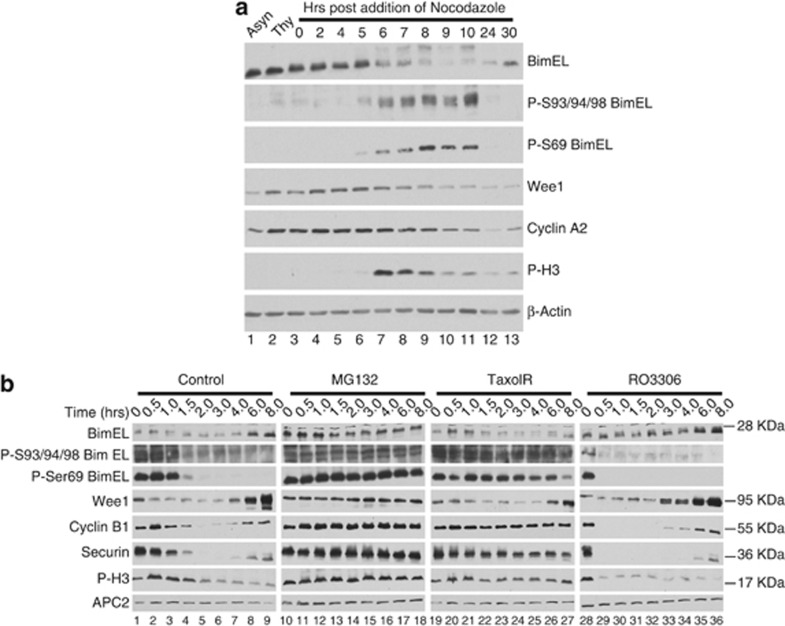
Figure 2.
BimEL is phosphorylated and degraded at mitosis in a mechanism independent of the spindle assembly checkpoint. (a) Immunoblot analysis of 293T cells synchronized as in Figure 1a with the exception that cells were left in nocodozole for the indicated times. Immnoblots were performed for total BimEL and phosphorylated BimEL on serine-69 (P-S69) and serines 93/94/98 (P-S93/94/98). Immunoblots for two other proteins known to be degraded during mitosis, wee1 and cyclin A2, were performed. Anti-phospho histone H3 (P-H3) is included as a marker for onset of mitosis and β-actin as loading control. (b) Immunoblot analysis of HeLa cells synchronized at mitosis as in Figure 1a and released into four different conditions: normal media (control), MG132 (10 μM), Taxol (100 nM), and RO3306 (10 μM). Whole-cell extracts were prepared at the indicated times post release. Immnoblots were performed for total BimEL, P-S69, and P-S93/94/98. Immunoblots for three other proteins known to be degraded during mitosis, Wee1, cyclin B1, and Securin, were performed for comparison. P-H3 is included as a marker for onset of mitosis and APC2 as loading control