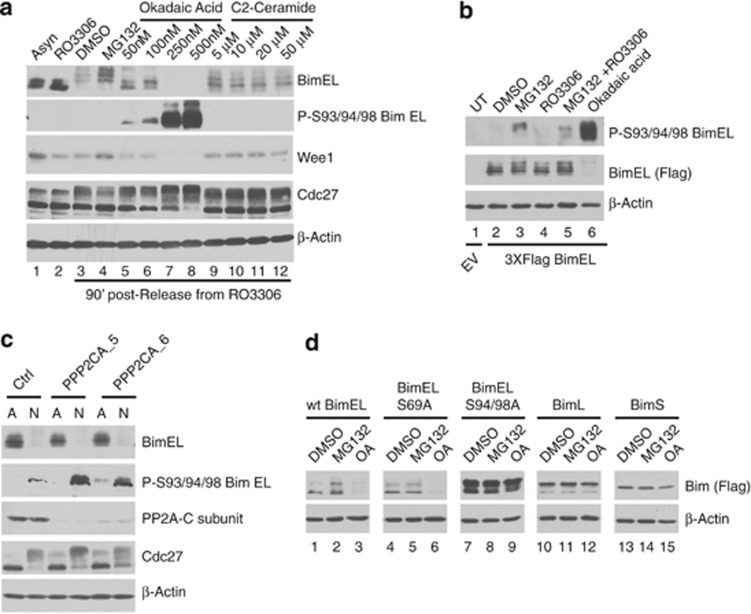
Figure 5.
PP2A dephosphorylates BimEL on Ser93/94/98 and prevents degradation. (a) HeLa cells were synchronized in G2/M with RO3306 and released into fresh media plus vechicle (DMSO), MG132, OA, or C2-ceramide at the indicated concentrations and harvested 90 min post release. Whole-cell extracts were analyzed by immunoblot to detect endogenous BimEL, phosphorylated BimEL on serines 93/94/98 (P-S93/94/98), and Wee1. Cdc27 immunoblots were used to confirm mitotic state of cells and β-actin as loading control. (b) 293T cells were transfected with wild-type (WT) Flag-tagged BimEL or left untransfected (UT). Cells were synchronized in mitosis as in Figure 1 and released into vehicle control (DMSO), MG132 (10 μM), RO3306 (9 μM), and OA (500 nM) as indicated. IP was performed using anti-FLAG antibody (BimEL) and immunoblot analysis used to detect IPed BimEL (FLAG) and P-S93/94/98. β-Actin immunoblot was used as the input control for IPs. (c) Knockdown of the PP2A catalytic subunit in HeLa cells using two different siRNA PPP2CA_5 and PPP2CA_6. A non-silencing siRNA (Ctl) was used as control. Immunoblot analysis was performed against endogenous BimEL, phosphorylated BimEL (P-S93/94/98), and the PP2A-C subunit. Cdc27 immunoblots were used to confirm mitotic state of cells and β-actin as loading control. (d) 293T cells were transfected with WT Flag-tagged BimEL, BimL, BimS, or phosphorylation site mutants as indicated. Transfected cells were synchronized in mitosis as shown in Figure 1a and treated with vehicle control (DMSO), MG132 (10 μM), or OA (500nM) as indicated. Whole-cell extracts were analyzed by immunblot using anti-Flag to detect Bim and β-actin as loading control