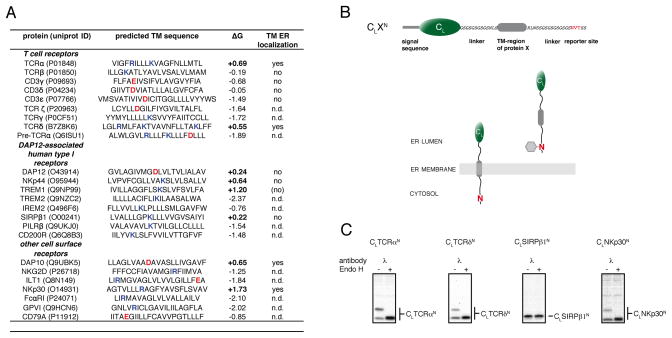
Figure 1. TM sequences of low hydrophobicity can enter the ER lumen.
(A) Analysis of TM sequences of human single pass cell surface receptor subunits whose assembly is focused on intramembrane polar residues. TM sequence assignments were taken from the uniprot database (www.uniprot.org). ΔG values were calculated according to (Hessa et al., 2007) (http://dgpred.cbr.su.se/). Positive ΔG values predict an unfavorable free energy of membrane integration (bold). Basic residues are highlighted in blue, acidic residues in red. TM regions that were tested experimentally for lumenal localization are indicated as yes/no, and those not examined are described as not determined (n.d.). The bracketed no for TREM1 indicates a very weak band detected for the C-terminally glycosylated species. Data for TCRβ and CD3γ, δ and ε are derived from Figure 2.
(B) Top: schematic outline of the reporter construct used to monitor ER-import of TM sequences. It is composed of the non-glycosylated immunoglobulin λ light chain CL domain connected by flexible linkers and short amino acid stretches derived form the TCR α-chain to the TM sequence of interest followed by a C-terminal glycosylation site (NVT, marked in red). Bottom: A cartoon showing that membrane integration of the reporter construct would not allow its C-terminal NVT site to become glycosylated, whereas complete ER import would expose it to the ER glycosylation machinery allowing it to become glycosylated (shown as grey hexagon).
(C) COS-1 cells transfected with the indicated constructs were metabolically labeled and cell lysates were immunoprecipitated with anti-mouse λ antiserum. Precipitated material was split and treated with or without EndoH prior to SDS-PAGE analysis. See also Figure S1.