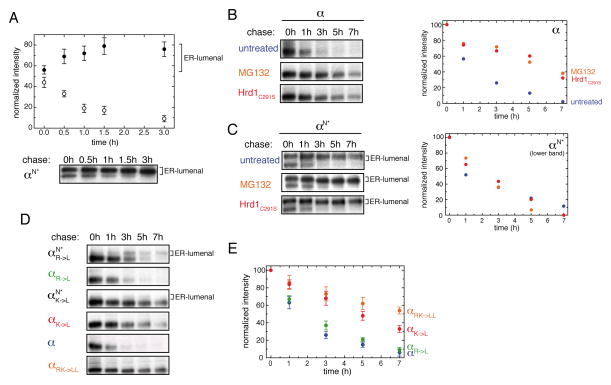
Figure 4. Degradation of the TCR α-chain correlates with localization of its TM region into the ER lumen.
(A) Quantification of the two glycospecies for αN*. Cells expressing the αN* construct were pulse-labeled and chased for the indicated times before immunoprecipitation and SDS-PAGE analyses (Bottom). Signal averages for the ER-lumenal, C-terminally glycosylated species are shown as closed circles, data for the species not C-terminally glycosylated are indicated with open circles (n=4±SD) (Top). Note that in this and most subsequent experiments a construct designated as αN* was used instead of αN (for details see Figure S4A).
(B) Inhibition of α-chain degradation was carried out by addition of MG132 (orange) or co-expression of Hrd1C291S (red) as indicated and compared to the degradation of the α-chain in the absence of any treatment (blue). Quantifications are shown on the right.
(C) Disappearance of the lower, not C-terminally glycosylated band for αN* was measured and quantified under the same conditions as in (B). Quantifications are shown on the right.
(D) Pulse-chase experiments were performed for different α-chain constructs containing either a single, none, or both TM basic residues replaced by Leu as indicated. For mutants with single TM basic residue replaced, ER-lumenal localization was additionally assessed by the αN* reporter construct. Species glycosylated at their C-terminal reporter site are indicated with “ER-lumenal” on the side.
(E) Quantifications (n=3±SD) of the degradation kinetics for the wt α-chain (blue), αRK->LL (orange), αR->L (green) and αK->L (red). See also Figure S4.