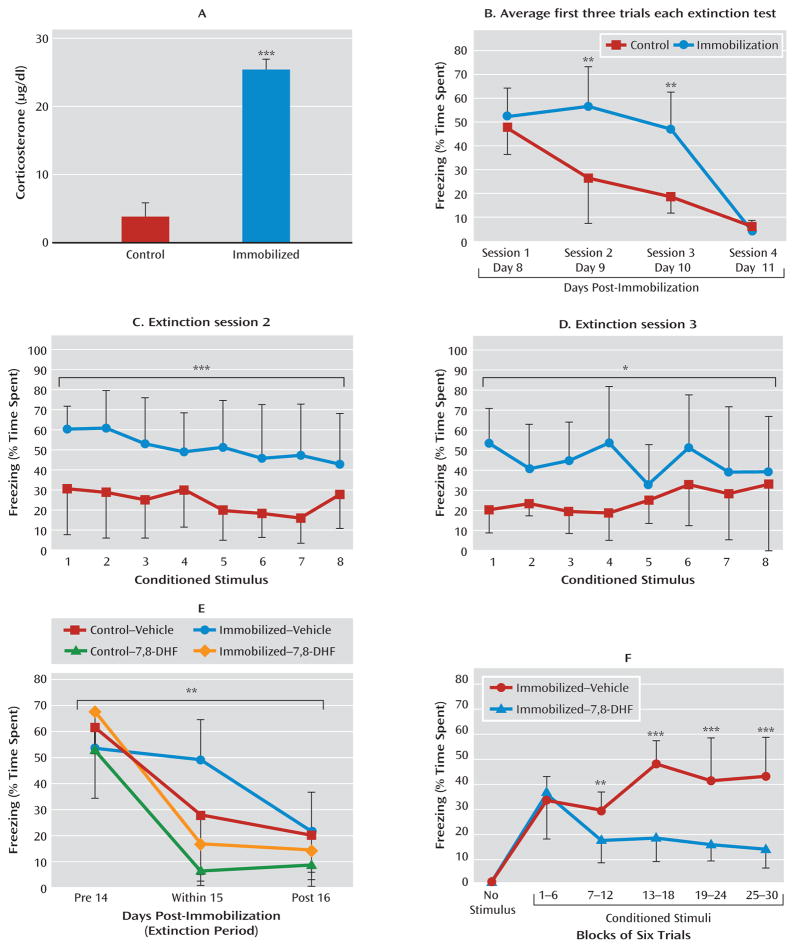
FIGURE 4. Effect of 7,8-Dihydroxyflavone (7,8-DHF) on Extinction in a Traumatic Stress Modela.
a Mice underwent 2-hour immobilization 6 days before the first session of fear conditioning, followed by repeated sessions of extinction. Panel A shows that shortly after immobilization, there is a robust activation of the hypothalamic-pituitary-adrenal axis, as demonstrated by a greater mean acute level of plasma corticosterone relative to control mice. In panel B, the immobilized group showed delayed extinction in analyses of conditioned freezing in extinction sessions 2 and 3 (panels C and D), whereas both groups had equivalent low levels of freezing by session 4, suggesting a delay in extinction following immobilization stress. (In panels A–D, ***p<0.001, **p<0.01, *p<0.05 between control and immobilization groups.) In panel E, animals also showed impaired extinction when fear-conditioned 12 days after immobilization stress. Because of the delayed nature of extinction in immobilization-treated animals, we examined the last session to compare differential freezing during pre-extinction, within-session extinction, and post-extinction sessions across groups (with and without immobilization, and with and without 7,8-DHF). Mice that were given 7,8-DHF prior to extinction showed a significant drug-by-session effect of enhancement of extinction across sessions (**p<0.01 session-by-group interaction between pre-extinction, within-extinction, and post-reinstatement). Panel F shows that 7,8-DHF was associated with decreased freezing in the within-session extinction trials in animals with a prior history of immobilization. Freezing (average of six trials within each bin) is shown during within-extinction session, 1 hour after administration of 7,8-DHF or vehicle (***p<0.001, **p<0.01 for the immobilization-vehicle compared with immobilization-7,8-DHF groups). Error bars indicate standard deviations.