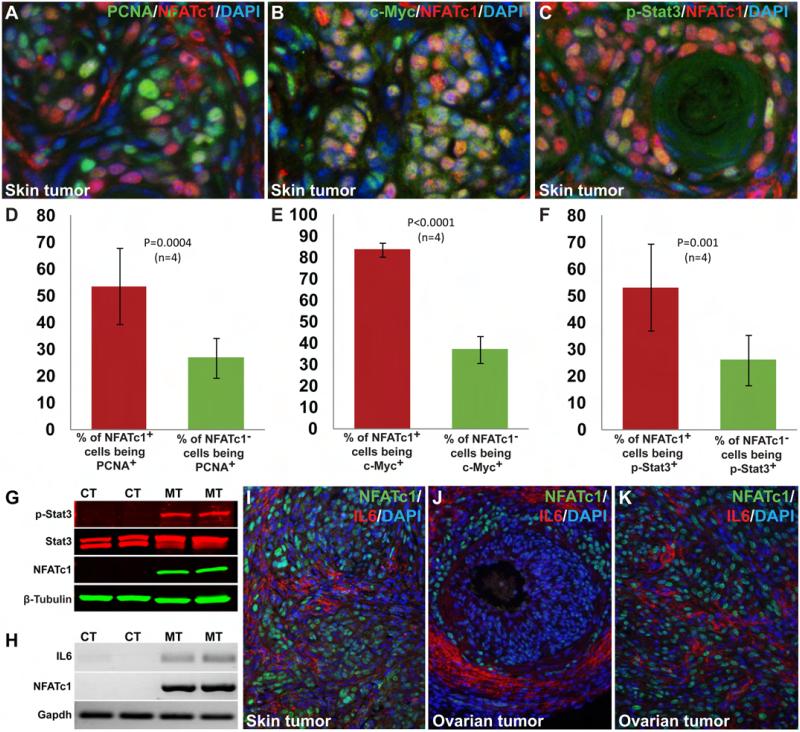
Fig. 4. High level of heterogeneity in the tumors and the non-cell autonomous promitogenic effects of NFATc1 activation.
Immunostaining using NFATc1 and PCNA as well as the subsequent statistical analyses (two-tailed t-test) indicated that a high percentage of NFATc1+ cells were actively proliferating (53.67±14.22%). The percentage of proliferating NFATc1− cells (26.92±7.43%, P=0.0004, n=4), albeit smaller, was very substantial (A, D). NFATc1 activation led to significant upregulation of c-Myc in the tumor tissue (B, E). 83.74±3.17% of the NFATc1+ cells expressed c-Myc, whereas only 37.07±6.42% NFATc1− cells were c-Myc+ (P<0.0001, n=4) (E). Double immunostaining with NFATc1 and phospho-Stat3 antibodies showed higher percentage of NFATc1+ cells with nuclear Stat3 (53.14±16.23%) than NFATc1− cells with nuclear Stat3 (26.18±9.42%, P=0.001, n=4) (C, F). Western blot analysis of normal skin lysates from control mice and skin tumor from mutant mice was done by using an antibody against Stat3 (revealing the level of Stat3 proteins regardless of phosphorylation status) and an antibody against phospho-Stat3 (Tyr705, revealing the level of activated Stat3). The results showed activation of Stat3 specifically in NFATc1-induced skin tumors while the level of total Stat3 was unchanged (G). NFATc1 activation specifically up-regulated IL6 in the tumors as revealed by RT-PCR (H). IL6 upregulation was also detected by immunostaining in skin (I) and ovarian tumors (J-K). CT; control, MT; mutant.