Abstract
Mexico has experienced changes in its demographic and epidemiologic profile accompanied by recent changes in nutrition and income. Thus, the old and the young have experienced very different environments. Using data from the Mexican National Health Nutrition Survey 2006, we examine age and sex differences in physiological status and dysregulation and assess how socioeconomic factors associate with variability in biological indicators of health. Results indicate that young people have experienced better physical development as evidenced by their being taller and having less stunting. There is currently little under-nutrition in Mexico, but there is evidence of over-nutrition as indicated by high prevalence of overweight across the age range. Physiological dysregulation across multiple systems is higher in Mexicans than Americans across all ages. Mexicans have: higher levels of blood pressure, plasma glucose, and especially for women, dysregulated cholesterol and higher body weight. Low education is associated with both being stunted and overweight, and with adverse levels of HDL cholesterol and more physiological risk factors. Rural dwelling males are less likely to be overweight as are females living in poor states. Living in a poor state among females and having rural residence among males is associated with a higher number of high-risk factors. Overweight is a strong predictor of hypertension. Age differences in indicators of physiological development suggest that the epidemiological and demographic transitions in Mexico were accompanied by improved physical development; however, increases in nutrition may have reached a point of diminishing returns as Mexico switched from a state of under-nutrition to over-nutrition.
Keywords: biomarkers, overweight, blood pressure, cholesterol, plasma glucose, education, Mexico
Introduction
Countries in Latin America have experienced recent changes in their demographic and epidemiologic profiles caused by or accompanied by even faster changes in nutrition and income. Nutritional and income improvements may have initially resulted in better physiological development and stronger resistance to infection. These changes have led to major shifts in national patterns of health and disease because infection has diminished as a cause of death and morbidity. Now chronic conditions, such as diabetes and cardiovascular disease, are becoming more prevalent in the region and are currently the leading causes of death in the adult population, while infectious diseases remain a latent reminder of the recent past (World Health Organization 2012). Moreover, in recent years several risk factors for chronic disease seem to be on the rise with an increasing number of people being afflicted by overweight and obesity (Popkin 2006). These increases in body weight are one dimension of the physiological change that is occurring in middle-income countries like Mexico.
Until recently, most of the research on national patterns of health in low- and middle-income countries has relied on self-reported health indicators. Although important, these studies are limited in their ability to increase our understanding of underlying processes leading to disease, disability and death. However, in recent years, a number of countries have collected biological data in large population-based surveys which allow us to get a more comprehensive understanding of physiological dysregulation and the processes determining population health. In this paper we examine a rich set of measured biological data from Mexico, a country where epidemiologic, demographic and income change have been dramatic and rapid. This allows us to identify salient demographic and socioeconomic characteristics linked to physiological status in the adult Mexican population and to examine differences in physiological status across age in this population where the old and the young have lived in very different environments. The underlying disease process over the life course in these cohorts has affected their current physiological status; thus, we hypothesize that earlier-born cohorts of Mexican adults will have residual effects of their earlier exposure to harsher conditions. Age differences in physiological dysregulation associated with chronic disease reflect both age and cohort experiences; as well as current and past socioeconomic status and behaviors, as these factors are indicators of differential lifetime exposure to risk for physiological dysregulation.
Demographic and epidemiologic changes and their links with current national patterns of health and disease in Mexico
Large declines in mortality rates experienced after 1940 reflect the major successes of medical interventions and improved standards of living in Mexico. For example, life expectancy at birth increased by 50% between 1940 and 1970, from 40.5 years in 1940 to 61.5 years in 1970 (Camposortega Cruz 1997), and subsequently increased by 23% to 75.4 years from 1970 to 2010 (Consejo Nacional de Población 2010). Of particular relevance were the major declines in infant and child mortality which dominated increases in life expectancy throughout the 20th century (Aguirre 1999; Palloni & Wyrick 1981).
Because of the changes in living standards and medical interventions, age subgroups of the population have had distinct life course experiences. On the one hand, people aged 60 or older in 2000 represent those who survived under adverse childhood circumstances where resistance to and recovery from infectious disease was primary for survival. Now people of this age are living their senior years where chronic conditions predominate (Samper-Ternent et al. 2012). Thus older cohorts may experience consequences of both their earlier exposure to a highly infectious environment as well as their later exposure to increasing risk factors for chronic disease (Montez & Hayward 2011; Warner & Hayward 2006). For instance, previous research shows that the Mexican elderly population has a high prevalence of diabetes and obesity (Palloni et al. 2003; Wong, Espinoza, & Palloni 2007).
On the other hand, people who were younger than 60 at the turn of the 21st century grew up in a childhood disease environment that was rapidly improving throughout their lives. Several mechanisms have been proposed linking changes in childhood conditions with adult health and disease (see Montez & Hayward 2011 for a review). Briefly, the fetal origins hypothesis posits that experiences early in the developmental period (e.g. in utero) can compromise organ development leading to higher likelihood of developing disease in adulthood (Barker 1997). Others argue that early life conditions may have indirect influences on adult health because they are one source of the accumulation of detrimental experiences over the life course which is the driving mechanism linking early and late life health (Ben-Shlomo & Kuh 2002). Finally, the inflammation hypothesis states that reduction in infectious and communicable diseases throughout life would reduce inflammation leading to less deterioration with age through this basic mechanism of age related change (Crimmins & Finch 2006; Finch 2007). All of these mechanisms would predict improving health in later-born Mexican cohorts. Because these changes have occurred over the lives of these cohorts, we hypothesize that we will observe differences by age in indicators of growth and development as well as in some aspects of physiological status as the result of differential exposure by age or cohort to epidemiological and socioeconomic conditions.
Evidence of change in some aspects of physiology in the Mexican population is provided by the increasing prevalence of overweight and obesity which increased between 1988 and 1999 by 47% and 160%, respectively, among women aged 18 to 49 in Mexico (Rivera et al. 2002). Similarly, the prevalence of diabetes, heart disease and hypertension increased over the same period (Rivera, et al. 2002) with particularly high prevalence of hypertension and overweight among people younger than 40 (Beltrán-Sánchez et al. 2011).
Dietary changes and their link with current national patterns of physiology and health risk factors in Mexico
In recent decades, people in many countries have experienced dramatic changes in their physiological status as a result of major shifts in diet, growing concentration of jobs requiring low energy expenditure, and environmental change (Crimmins et al. 2013; Fogel & Costa 1997; Popkin 2006). The positive consequences of improved nutrition have been credited with a major role in reducing infant and childhood mortality during the demographic transition (McKeown, Record, & Turner 1975); the increased nutrition is thought to improve both resistance to disease and resources available for recovery from infection. However, increases in nutrition may have reached a point of diminishing returns as countries switch from a state of under-nutrition (low calorie consumption) to over-nutrition (higher than needed calorie consumption).
Mexicans have not escaped this trend and the consequences are beginning to emerge. For instance, major increases in both fat intake and purchase of sugars and refined carbohydrates have been reported along with a decline in the purchase of fruits and vegetables (Rivera, et al. 2002; Rivera et al. 2004). These dietary changes have swept throughout Mexico. In the 1990s, the more urban areas, typically located in the Northern region and Mexico City, had a greater increase in fat consumption relative to the less urban areas of the South (Rivera, et al. 2004); however, in recent years, this pattern of high fat consumption has become more homogeneous across regions as a result of increasing fat consumption rates in the South (Barquera et al. 2006). Additionally, there have been recent changes in traditional dietary habits in that people are more likely to eat away from home (Barquera, et al. 2006) and the traditional Mexican diet based on corn and beans, typical of rural areas in the mid-1980s, has been replaced by industrially produced foods including refined carbohydrates and sugar-sweetened drinks by the late 1990s (e.g., soda, fruit juices, etc.) (Aguirre-Arenas, Escobar-Pérez, & Chávez-Villasana 1998).
Changes in physiology that may be related to these dietary changes have been observed even among the young. For example, the prevalence of hypertension was already about 11% in a sample of junior high school (aged 12–16 years) students in Mexico City (Juarez-Rojas et al. 2008) and increasing body weight has also been observed among adolescents, particularly in urban places (Yamamoto-Kimura et al. 2006).
In this paper we examine the age and sex pattern of differences in physiological status and dysregulation to provide a picture of population health across the adult population as well as some hints of how health has changed over time. In order to provide a reference for the Mexican data, we compare the levels of these indicators to those of a nationally representative sample of the United States. The United States is recognized as having relatively poor health among high income/low mortality countries (Crimmins, Preston, & Cohen 2011). We also examine how socioeconomic factors associate with variability in biological indicators of health within the adult Mexican population. We assume that the changes occurring in Mexican society over recent decades have differentially affected those who are more educated and urban. As a result of the intertwined dynamics in demographic and epidemiologic circumstances accompanied by recent dietary changes, we expect differences in the health profile of the Mexican population to vary by age, gender, education, and rural residence. We hypothesize that younger persons will have experienced better physical development as evidenced by their being taller and having less stunting resulting from improving levels of infection and nutrition. We also hypothesize that we will observe reductions in under-nutrition, particularly for the young. On the other hand, we expect evidence of increases in over-nutrition across all ages. We also expect differences in the cardiovascular and metabolic profiles by age with a larger number of high-risk factors among the old.
Data
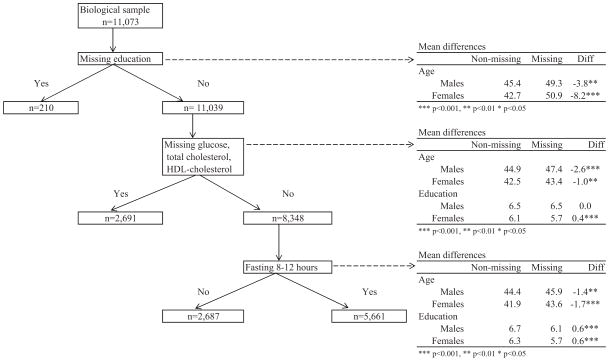
We used data from the nationally representative Mexican National Health and Nutrition Survey 2006 (ENSANUT 2006). This is based on a probabilistic, multistage, stratified cluster sample design including 47,152 households (see Olaiz-Fernández et al. 2006 for more details). From each household three randomly selected individuals were interviewed: under age 10, aged 11–19, and aged 20 or older. Blood was collected from randomly selected participants that were instructed not to eat any solid or liquid food prior to their blood draw. The time of last food eaten was recorded in the questionnaire. The adult sample includes 45,241 people aged 20 or older of whom 11,073 provided blood samples (Figure 1). We eliminated 210 people with missing values in education (n=11,039). People with missing education were, on average, significantly older. There were an additional 2,691 people with missing values in glucose, HDL-cholesterol and total cholesterol (n=8,348). Statistical analyses using two-tailed t-tests indicate that those with missing biomarker data are significantly older for both males and females and have significantly lower mean education level for females but no differences for males. Additionally, in order to use plasma glucose levels, the sample was restricted to those who fasted between 8 and 12 hours eliminating 2,687 people. Thus, the final analytical sample contains 5,661 individuals. Statistical tests indicate that people who fasted tended to be younger and with a slightly higher level of education. Sub-sampling procedures and laboratory methodological details are described elsewhere (Barquera, Campos-Nonato, Carrión-Rábago, et al. 2010; Olaiz-Fernández, et al. 2006). For comparative purposes, we present data for the same indicators from the National Health Nutrition and Examination Survey (NHANES) 2001–2006 from the United States including all race/ethnicity groups (Centers for Disease Control and Prevention (CDC) & National Center for Health Statistics (NCHS) 2012).
Figure 1.
Sample selection for the Mexican adult population aged 20 or older with biomarker indicators of health and socioeconomic and demographic characteristics: ENSANUT 2006
Note: Mean differences estimated from two-tailed t-tests
Measures
We included measures of indicators of body size, growth and development: total height, and stunting or short height. We also include body mass index which combines information on height and weight and we examine low BMI as an indicator of current under-nutrition (low calorie consumption) and high BMI or overweight as an indicator of over-nutrition (higher than needed calorie consumption). We also include measures of physiological dysregulation based on measures of cardiovascular and metabolic functioning: blood pressure, total cholesterol, high-density lipoprotein (HDL) cholesterol, and plasma glucose. Laboratory methodology and measurement procedures for ENSANUT 2006 are described elsewhere (Olaiz-Fernández, et al. 2006). Briefly, participants removed their shoes and upper garments before measures of height and weight were taken. Height was measured using a stadiometer to the nearest 0.5 cm and body weight was measured on an electronic scale calibrated daily and recorded to the nearest 0.1 kg (Barquera et al. 2009). Blood pressure was measured twice with at least five minutes between measures while the participant was seated. A trained nurse took the measures in the dominant arm using a mercury sphygmomanometer on two different readings and we use the average of the two measures (Barquera, Campos-Nonato, Hernández-Barrera, et al. 2010). Total cholesterol was determined using enzymatic hydrolysis and oxidation. HDL-cholesterol was measured using an enzymatic colorimetric direct method (Aguilar-Salinas et al. 2010). Data collection, laboratory methodology and measurement procedures for the NHANES data used for the U.S. are comparable and are described elsewhere (Centers for Disease Control and Prevention (CDC) & National Center for Health Statistics (NCHS) 2012).
High-Risk Cut Point for Biological Indicators
Following prior research in this area, we use clinically or research defined cutoffs to divide the sample into those with indicators of poor health or high-risks for subsequent disease and mortality and those not in the risky range. Stunting, which is an indicator of poor early life growth due to either high levels of disease or poor nutrition, is defined by having height < 1.55 meters for men and <1.40 meters for women. Body mass index (BMI) is estimated as the ratio of weight (measured in kilograms) over the square of height (measured in meters), and then categorized into overweight (BMI ≥ 25), underweight (BMI ≤ 18.5) and normal weight (18.5 < BMI < 25).
Hypertension is indicated by average measured blood pressure above the clinically recognized threshold (systolic ≥ 140 mmHg or diastolic ≥ 90 mmHg) or by taking medication for high blood pressure, which is self-reported in the interview. We defined high cholesterol as having total cholesterol ≥240 mg/dL, low HDL-cholesterol as ≤ 40 mg/dL, and high fasting glucose as ≥110 mg/dL. Similar cut-off points where used for the ENSANUT and NHANES data.
Physiological Dysregulation Summary Risk Score
We also created a summary measure of biological risk for chronic disease that corresponds to the number of cardiovascular and metabolic factors an individual has in the high-risk range. Summary indicators such as the metabolic syndrome (Mitka 2004), the Framingham risk score (Lloyd-Jones et al. 2004), and allostatic load (Seeman et al. 2001) have been used in many studies to predict chronic disease, disability, and death. The use of a simple count measure such as we are using has been shown to be a useful approach to predicting health outcomes (Gruenewald et al. 2006). An advantage of a simple sum of high-risk factors is its simplicity when interpreting results across populations (Crimmins, Kim, & Seeman 2009). Our summary risk score for each individual ranges from 0 (none) to 6 (all) where one point is given to each indicator of physiological dysregulation in the high-risk range (high systolic, high diastolic blood pressure, high total cholesterol, low HDL cholesterol, high plasma glucose, and obesity).
Socioeconomic and demographic indicators
We also included education, rural residence, age and gender in the analysis to examine variability in physiological status by demographic and social characteristics. Education is categorized into low (9 or fewer years of completed schooling) and high (10 or more years of completed schooling). Rural residence means living in a locality with fewer than 2,500 inhabitants. We additionally created an indicator of living in what we call a “State with poor epidemiological conditions.” This means one resides in one of the 11 states which experienced a more recent epidemiological transition, infant mortality is currently around 20.0 or higher (CONAPO 2012), and there is a low GDP per capita and high poverty level (Consejo Nacional de Evaluación de la Politica de Desarrollo Social 2007). The 11 (out of the 31) states include: Campeche, Chiapas, Guerrero, Hidalgo, Michoacán, Oaxaca, Puebla, San Luis Potosí, Tabasco, Veracruz, and Zacatecas.
Methods
First, we estimate the prevalence by age and sex of indicators of physiological status as indexed by individual indicators and a summary measure for Mexico and the United States. In the second phase of our analyses, we estimated a series of regression models for the Mexican sample including age, education, rural residence and poor State to assess the association of these covariates with each biological indicator. Models are estimated separately for each biological marker using logistic regression for dummy indicators (e.g., stunting, underweight, overweight, and presence of high-risk levels of biological markers) and linear regression for total height and the summary measure. All analyses take into account the complex sampling design of the surveys. We used Stata 12.0 in all estimates.
Results
Indicators of Physical Development and Nutrition
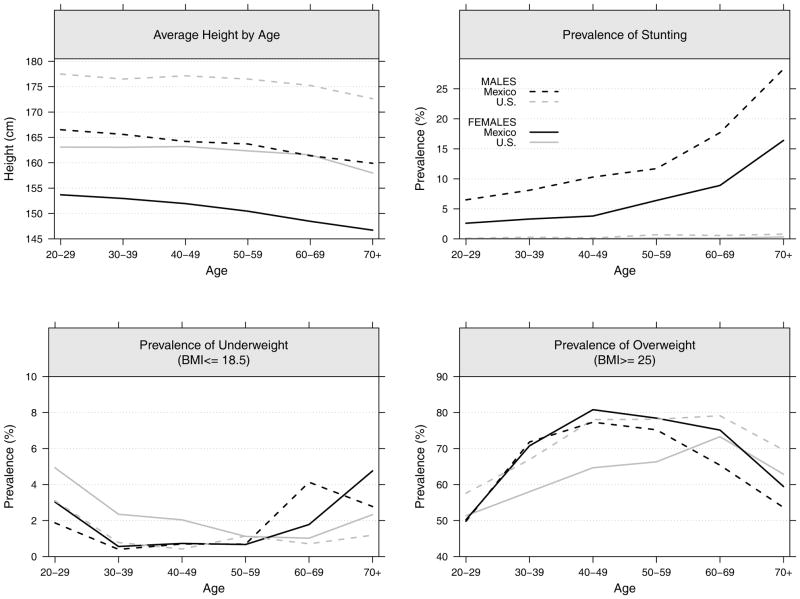
We first present age-specific estimates by gender for average total height, and the prevalences of stunting, underweight, and overweight. We assume that these indicators of body size are influenced by both past and current nutrition and illness (Figure 2). The pattern of age differences in height shows that the Mexican population has a steeper increase across younger ages indicating increasing height among younger cohorts. It also indicates that the U.S. has a flatter curve at ages up to 50 indicating a cessation in the trend toward increasing height in more recent cohorts. The U.S. and Mexico are getting closer in height in younger cohorts; although height in the U.S. exceeds Mexican average height at all ages for both women and men.
Figure 2.
Average total height, and prevalences of stunting, underweight and overweight for the adult population aged 20 or older by sex in Mexico and the U.S.: ENSANUT 2006 and NHANES 2001–2006
Because of the epidemiological and nutritional history, stunting is virtually nonexistent in the U.S. In Mexico, stunting is more prevalent among older cohorts with almost a third of men 70+ being stunted while only 15 percent of women are in this category. This suggests that their physical development was probably suboptimal because of inadequate nourishment of usable nutrients due to infection or poor diets. The marked decrease at younger ages in stunting, reaching low levels among 20–29 years old, reflects better childhood environments and better physical development of younger Mexican cohorts.
Under-nutrition (i.e., underweight), a marker of current poor nutritional status, is relatively rare in both Mexico and the U.S.; although, it is more common among young American women and older Mexican women. On the other hand, overweight is very high in both Mexico and the U.S. Mexican women showed a higher prevalence of overweight up to age 60 than their American counterparts, while Mexican men have a lower prevalence among the youngest (20–29 years) and oldest (50+) groups. In the American population, the most overweight group is among those 60 years of age; among Mexicans, the highest levels of overweight occur 20 years earlier, or among those in their 40s.
Regression results associating these indicators of body size and stature with socioeconomic conditions and demographic characteristics in Mexico are shown in Table 1. Older people are more likely to be shorter and stunted when the additional controls are in the equation. People aged 30–69 are heavier than those in their 20s. Low education is significantly related to three of the adverse health states: shorter height, stunting and overweight. For instance, those with low education are about 5 times more likely to be stunted but are significantly less likely to be underweight. Education shows a strong association with overweight suggesting that females with low education are about twice as likely as their counterparts with more education to be overweight. Similarly, people living in a state with a later epidemiological transition or poor state are also more likely to be shorter or stunted (with odds of stunting twice as high). Additionally, rural dwelling males are less likely to be overweight as are females who live in poor States. There is no significant relationship between being stunted and being overweight or underweight.
Table 1.
Association between Total Height, Stunting, Underweight and Overweight with Age, Education, Rural Residence, an Indicator of “Poor” State for the Mexican Population aged 20 or older: ENSANUT 2006
| Covariates | Total Heighta | Stuntingb,c | Underweightc BMI ≤ 18.5 | Overweightc BMI ≥ 25 | ||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | Males | Females | |
| Age (ref=20–29) | ||||||||
| 30–39 | −1.20 [−2.92,0.51] | −0.78 [−1.71,0.15] | 1.79 [0.74,4.31] | 1.70 [0.59,4.95] | 0.40 [0.05,3.36] | 0.06*** [0.02,0.22] | 2.62*** [1.66,4.12] | 2.57*** [1.78,3.73] |
| 40–49 | −1.81* [−3.35, −0.27] | −1.60** [−2.62, −0.58] | 2.19 [0.95,5.06] | 1.18 [0.46,3.03] | 0.60 [0.11,3.44] | 0.02*** [0.00,0.13] | 3.40*** [2.20,5.27] | 4.94*** [3.37,7.25] |
| 50–59 | −3.09*** [−4.89, −1.30] | −2.54*** [−3.73, −1.36] | 3.50** [1.43,8.60] | 2.43 [0.91,6.52] | 0.37 [0.08,1.76] | 0.07** [0.01,0.35] | 3.39*** [2.06,5.60] | 4.69*** [3.08,7.14] |
| 60–69 | −2.85*** [−4.50, −1.20] | −3.87*** [−5.07, −2.68] | 3.38** [1.35,8.49] | 6.65*** [2.74,16.16] | 0.99 [0.18,5.40] | 1.00 [1.00,1.00] | 3.59*** [2.10,6.12] | 3.00*** [1.79,5.05] |
| 70–+ | −6.02*** [−8.13, −3.91] | −5.84*** [−7.26, −4.41] | 7.75*** [3.07,19.57] | 14.35*** [6.34,32.50] | 1.52 [0.46,5.05] | 0.37 [0.09,1.47] | 1.58 [0.92,2.74] | 1.40 [0.80,2.45] |
| Low Education | −3.52*** [−4.76, −2.29] | −4.07*** [−4.96, −3.18] | 4.70*** [2.00,11.02] | 5.29* [1.06,26.52] | 0.36* [0.14,0.94] | 0.29** [0.11,0.74] | 1.46* [1.00,2.11] | 2.08*** [1.47,2.94] |
| Rural | −0.47 [−1.76,0.81] | −0.11 [−0.77,0.54] | 1.43 [0.84,2.44] | 0.89 [0.52,1.53] | 1.76 [0.55,5.59] | 2.67 [0.85,8.39] | 0.65** [0.48,0.89] | 0.89 [0.69,1.15] |
| Poor State | −3.71*** [−4.70, −2.72] | −2.63*** [−3.29, −1.98] | 2.46** [1.43,4.23] | 2.36** [1.30,4.30] | 0.30* [0.10,0.93] | 0.41 [0.11,1.48] | 0.95 [0.71,1.27] | 0.77* [0.60,0.99] |
| Stunted | -------- | -------- | -------- | -------- | 0.29 [0.06,1.34] | n.a.d | 0.74 [0.44,1.25] | 0.77 [0.37,1.59] |
p<0.001,
p<0.01
p<0.05
Coefficients from OLS regression;
Stunting is defined as total height <1.55 meters for males and < 1.40 meters for females;
Coefficients from logistic regression;
Not enough sample size to estimate this coefficient.
Note: Coefficient estimates and 95% confidence intervals (shown in brackets). All estimates are weighted taking into account the complex sampling design of the survey.
Indicators of Metabolic Dysregulation
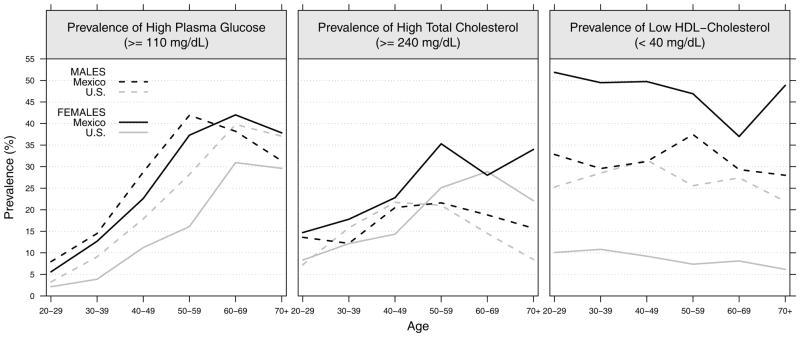
The age-specific gender prevalence of lipid dysregulation is indicated by high plasma glucose, high total cholesterol and low-HDL cholesterol (Figure 3). Mexican men have a higher prevalence of high plasma glucose than the U.S. up to age 60; for women the level for Mexicans is higher at all ages. The rise with age in high plasma glucose is stronger in Mexico than in the U.S. and males and females have more similar levels in Mexico.
Figure 3.
Prevalence estimates of high fasting plasma glucose (≥110 mg/dL), high cholesterol ((≥240 mg/dL) and low HDL cholesterol (<40 mg/dL) for the adult population aged 20 or older by sex in Mexico and the U.S.: ENSANUT 2006 and NHANES 2001–2006
The prevalence of high risk total cholesterol is similar between Mexican and American men; but Mexican women have higher total cholesterol than American women until the older ages. However, while the prevalence of low-HDL cholesterol is almost identical between Mexican and American males, Mexican females show about 5 times higher levels than American females.
Table 2 shows regression results linking socioeconomic indicators with cholesterol and plasma glucose levels in Mexico. Older females are more likely to have higher lipid levels; for men there are no significant differences by age in adverse levels of total or HDL cholesterol. Both older males and females have significantly higher plasma glucose levels; the increase with age through the 60s is quite marked with 60–69 year old males 12 times as likely, and females 8.5 times as likely, to have high plasma glucose as those in their 20s. Education is not significantly associated with high total cholesterol. However, females with low education are significantly less likely to have low HDL cholesterol but more likely to have high plasma glucose levels relative to those with more education. In addition, females but not males living in a poor state are less likely to have worse lipid levels, and males residing in poor state are less likely to have high plasma glucose. There is also evidence that nutritional status associates with cholesterol levels and plasma glucose. Stunting is linked with worse HDL cholesterol among males. On the other hand, overweight is consistently associated with lower likelihood of high total cholesterol and low HDL cholesterol, although it also increases the odds of having high plasma glucose with a significant link among women.
Table 2.
Association between High Total Cholesterol, Low-HDL Cholesterol, and High Plasma Glucose with Age, Education, Rural Residence, and an Indicator of “Poor” State for the Mexican Population aged 20 or older: ENSANUT 2006
| Covariates | High Total Cholesterol ≥ 240 mg/dL | Low HDL Cholesterol ≤ 40 mg/dL | High plasma glucose ≥ 110 mg/dL | |||
|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | |
| Age (ref=20–29) | ||||||
| 30–39 | 0.94 [0.51,1.75] | 1.60* [1.01,2.55] | 0.81 [0.52,1.27] | 1.09 [0.78,1.52] | 1.88 [0.74,4.80] | 1.77 [0.76,4.13] |
| 40–49 | 1.51 [0.85,2.69] | 2.18** [1.37,3.46] | 0.80 [0.51,1.25] | 1.32 [0.93,1.87] | 5.60*** [2.27,13.82] | 3.89** [1.71,8.86] |
| 50–59 | 1.53 [0.83,2.80] | 4.83*** [2.98,7.83] | 0.97 [0.61,1.55] | 2.15*** [1.45,3.19] | 11.87*** [4.75,29.64] | 5.82*** [2.52,13.43] |
| 60–69 | 1.72 [0.87,3.38] | 3.73*** [2.15,6.47] | 0.95 [0.54,1.67] | 1.64* [1.05,2.54] | 12.16*** [4.86,30.48] | 8.50*** [3.66,19.74] |
| 70-+ | 0.96 [0.41,2.24] | 5.36*** [2.97,9.67] | 0.59 [0.32,1.08] | 1.67* [1.01,2.78] | 9.80*** [3.48,27.64] | 7.51*** [2.95,19.09] |
| Low Education | 0.85 [0.54,1.34] | 0.71 [0.49,1.02] | 0.93 [0.64,1.34] | 0.61** [0.45,0.83] | 1.07 [0.63,1.81] | 1.85* [1.08,3.18] |
| Rural | 1.03 [0.70,1.52] | 0.74* [0.55,0.98] | 1.43* [1.06,1.94] | 0.85 [0.67,1.07] | 0.62* [0.39,0.98] | 0.67 [0.43,1.03] |
| Poor State | 0.87 [0.61,1.23] | 0.59*** [0.45,0.76] | 0.92 [0.70,1.21] | 0.62*** [0.50,0.77] | 0.54** [0.35,0.82] | 1.12 [0.78,1.61] |
| Stunted | 1.67 [0.89,3.12] | 0.62 [0.28,1.33] | 2.15** [1.31,3.50] | 0.86 [0.48,1.55] | 1.15 [0.58,2.30] | 1.39 [0.70,2.78] |
| Overweight | 0.86 [0.59,1.26] | 0.90 [0.65,1.24] | 0.51*** [0.38,0.70] | 0.63*** [0.48,0.81] | 1.23 [0.78,1.94] | 1.87** [1.19,2.95] |
p<0.001,
p<0.01
p<0.05
Note: Coefficient estimates and 95% confidence intervals (shown in brackets) from logistic models. All estimates are weighted taking into account the survey sampling design.
Indicators of Cardiovascular Dysregulation
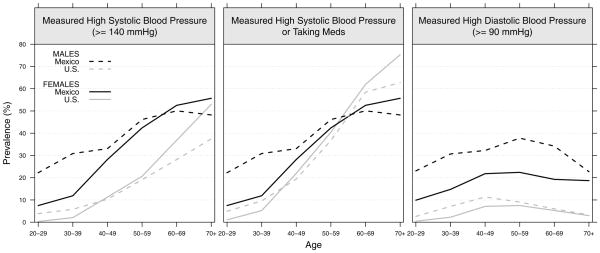
The prevalence of measured high systolic blood pressure, an alternative indicator of either high measured systolic blood pressure or use of medication to control blood pressure, and high diastolic blood pressure are shown in Figure 4. The prevalence of high systolic blood pressure is clearly higher for the Mexican population than in the U.S., with particularly worrisome rates among young adults (younger than 40). About one-third of Mexican males aged 30–50 have high systolic blood pressure. The prevalence rises very rapidly after age 30 among females and age 40 among males. When individuals who are taking medication to control their blood pressure are also considered, the pattern of national differences is quite different at the older ages. Because a large proportion of Americans use anti-hypertensive medication after age 40, the prevalence of medication or measured high systolic blood pressure is higher for Americans after age 60. Because the use of medication among Mexicans is almost negligible, a high proportion is clinically undiagnosed. This raises several concerns given the large proportion of young Mexicans with high systolic blood pressure. Differences are somewhat similar for high diastolic blood pressure although prevalence levels are much lower. Mexicans show a higher prevalence than their American counterparts with Mexican males having particularly high levels. About one-third of Mexican males aged 30–70 have high diastolic blood pressure.
Figure 4.
Prevalence estimates of high systolic blood pressure (≥140 mm Hg), high systolic blood pressure or taking anti-hypertensive medication and high diastolic blood pressure (≥90 mm Hg) for the adult population aged 20 or older by sex in Mexico and the U.S.: ENSANUT 2006 and NHANES 2001–2006
Regression results linking socioeconomic indicators with high blood pressure in the Mexican population are shown in Table 3. After age, the major risk factor for having high blood pressure is being overweight for both males and females; it approximately triples the likelihood among males. Stunting is significantly linked to less high systolic blood pressure among men and less high diastolic blood pressure among women using antihypertensives. High blood pressure does not seem to be significantly associated with education, rural living (one exception), or being in a state with a more recent epidemiological transition in this sample.
Table 3.
Association between High Systolic and Diastolic Blood Pressure with Age, Education, Rural Residence, an Indicator of “Poor” State and Indicators of Nutritional Status for the Mexican Population aged 20 or older: ENSANUT 2006
| Covariates | High SBP (≥140 mmHg) |
High SBP or Meds |
High DBP (≥90 mmHg) |
High DBP or Meds |
||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | Males | Females | Males | Females | |
| Age (ref=20–29) | ||||||||
| 30–39 | 0.88 [0.36,2.20] | 0.84 [0.37,1.87] | 1.00 [0.42,2.40] | 1.00 [0.51,1.97] | 1.18 [0.67,2.10] | 1.94* [1.07,3.52] | 1.24 [0.71,2.19] | 1.89* [1.10,3.26] |
| 40–49 | 2.59* [1.05,6.35] | 1.91 [0.89,4.09] | 2.90* [1.23,6.81] | 2.54** [1.31,4.93] | 1.62 [0.93,2.83] | 2.58** [1.40,4.73] | 1.73* [1.00,2.99] | 2.97*** [1.71,5.17] |
| 50–59 | 3.29** [1.34,8.05] | 8.42*** [3.88,18.25] | 4.96*** [2.14,11.52] | 11.21*** [5.72,21.97] | 2.24** [1.24,4.01] | 5.49*** [2.91,10.37] | 2.92*** [1.66,5.13] | 7.43*** [4.18,13.19] |
| 60–69 | 4.09** [1.64,10.18] | 14.04*** [6.24,31.61] | 8.30*** [3.45,19.94] | 25.01*** [12.27,50.97] | 1.62 [0.85,3.11] | 6.24*** [3.11,12.53] | 3.13*** [1.67,5.86] | 13.26*** [7.12,24.70] |
| 70–+ | 7.80*** [3.15,19.28] | 24.61*** [10.29,58.90] | 16.60*** [7.05,39.08] | 39.29*** [18.19,84.88] | 2.50** [1.26,4.95] | 7.51*** [3.66,15.43] | 6.49*** [3.52,11.97] | 16.90*** [8.71,32.79] |
| Low Education | 0.90 [0.53,1.55] | 0.97 [0.54,1.75] | 0.91 [0.56,1.48] | 0.86 [0.54,1.35] | 1.05 [0.69,1.59] | 0.79 [0.49,1.26] | 1.07 [0.72,1.60] | 0.88 [0.58,1.32] |
| Rural | 1.05 [0.72,1.54] | 1.38 [0.95,1.99] | 0.85 [0.59,1.23] | 0.99 [0.71,1.39] | 0.82 [0.60,1.13] | 1.18 [0.87,1.60] | 0.67* [0.49,0.92] | 0.85 [0.64,1.13] |
| Poor State | 1.29 [0.89,1.87] | 1.01 [0.71,1.43] | 1.15 [0.81,1.63] | 1.02 [0.74,1.40] | 1.02 [0.75,1.38] | 0.87 [0.65,1.16] | 0.94 [0.70,1.26] | 0.89 [0.67,1.16] |
| Stunted | 0.44** [0.25,0.78] | 0.67 [0.33,1.36] | 0.57* [0.33,0.99] | 0.59 [0.30,1.16] | 0.55 [0.30,1.02] | 0.56 [0.26,1.22] | 0.60 [0.34,1.07] | 0.50* [0.26,0.94] |
| Overweight | 2.78*** [1.79,4.32] | 1.32 [0.84,2.07] | 3.04*** [2.02,4.59] | 1.43 [0.98,2.08] | 2.87*** [1.90,4.33] | 2.14*** [1.38,3.32] | 3.18*** [2.17,4.65] | 1.99*** [1.37,2.88] |
p<0.001,
p<0.01
p<0.05
Note: Coefficient estimates and 95% confidence intervals (shown in brackets) from logistic models. All estimates are weighted taking into account the survey sampling design.
Summary risk indicator
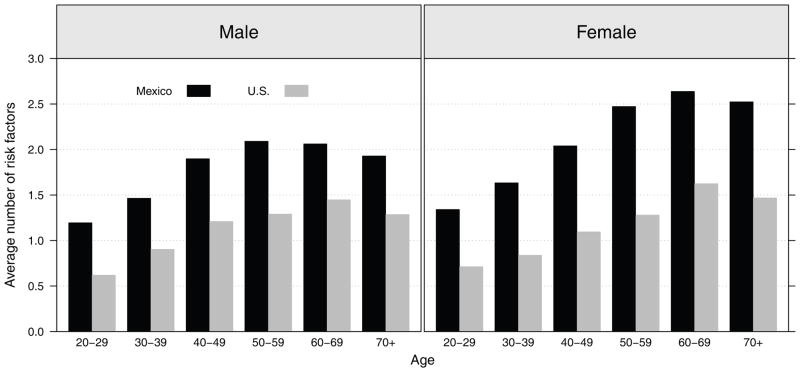
Summary risk scores of physiological dysregulation in the cardiovascular and metabolic markers by age and nationality are shown in Figure 5. Physiological dysregulation in the Mexican population is higher than that among Americans at every age, the differences tend to widen with age through ages in the 50s where they are maximal. The differences between American and Mexican women are particularly large. Analyses in the Mexican population linking these patterns with socioeconomic indicators (Table 4) show that the summary risk score is significantly higher among older males and females. Low education shows a significant link to higher risk scores among women; living in a poor state among females and having rural residence among males are also linked to higher scores.
Figure 5.
Average number of high-risk biological markers for the adult population aged 20 or older in Mexico and the U.S.: ENSANUT 2006 and NHANES 2001–2006
Note: A summary risk score is constructed for each individual ranging from 0 (none) to 6 (all) where one point is given to each biological marker in the high-risk range: high systolic, high diastolic blood pressure, high total cholesterol, low HDL cholesterol, high plasma glucose, and obesity. Numbers shown correspond to the average summary risk score.
Table 4.
Association between Summary Risk Score with Age, Education, Rural Residence, and Indicator of “Poor” State for the Mexican Population aged 20 or older: ENSANUT 2006
| Covariates | Summary Risk Score | |
|---|---|---|
| Males | Females | |
| Age (ref=20–29) | ||
| 30–39 | 1.31** [1.09,1.57] | 1.30*** [1.14,1.48] |
| 40–49 | 2.07*** [1.66,2.59] | 1.93*** [1.65,2.25] |
| 50–59 | 2.48*** [1.92,3.21] | 2.91*** [2.39,3.55] |
| 60–69 | 2.40*** [1.85,3.12] | 3.44*** [2.78,4.25] |
| 70-+ | 2.13*** [1.68,2.71] | 3.03*** [2.23,4.13] |
| Low Education | 1.04 [0.88,1.24] | 1.22** [1.05,1.41] |
| Rural | 0.71*** [0.61,0.83] | 0.92 [0.82,1.02] |
| Poor State | 1.01 [0.88,1.15] | 0.90* [0.81,0.99] |
p<0.001,
p<0.01
p<0.05
Note: Coefficient estimates and 95% confidence intervals (shown in brackets) from linear models. All estimates are weighted taking into account the survey sampling design.
Discussion
Differences by age in indicators of physiological development suggest that the epidemiological and demographic transition was accompanied by improved physical development. However, high levels of cardiovascular and metabolic dysregulation in Mexico relative to the United States suggest that changes in nutrition and activity over recent decades may now be having serious health consequences in the Mexican population. We expected differences in the health profile of the adult Mexican population to vary by age, gender, education, and rural residence. Our results support this hypothesis using measured indicators of four dimensions of health: physical development and nutrition, metabolic dysregulation, cardiovascular dysregulation, and a summary risk score.
First, we hypothesized that younger persons will have experienced better development as evidenced by their being taller and having less stunting resulting from improving levels of infection and nutrition. Our results indicate that stunting is currently more prevalent among older Mexican cohorts suggesting their physical development was probably suboptimal because of inadequate nourishment of usable nutrients due to infection. There is also marked decrease with age in stunting, reaching the lowest levels among 20–29 years old, reflecting better childhood environments and better physical development of younger Mexican cohorts. Second, we also hypothesize that there have been reductions in under-nutrition, particularly for the young. We found that under-nutrition is relatively rare in Mexico with the highest prevalence of about 4% occurring among older people (aged 60+).
On the other hand, we find evidence of over-nutrition, indicated by being overweight, across the age range. Overweight is highly prevalent in the adult Mexican population with about 70% of people aged 30–60 being overweight and about 60% of those aged 60+. Previous research shows high prevalence rates of diabetes and obesity among the elderly Mexican population in the early 2000s (Palloni, et al. 2003; Wong, et al. 2007); our research indicates that the high levels of overweight are occurring at young adult years. This pattern of decreasing under-nutrition and increasing over-nutrition has emerged very rapidly among low- and middle-income countries in recent decades (Popkin 2002, 2006). These may be related in that people’s diets and activities may have developed in an infectious environment. Because fighting infection is highly demanding of energy, people may have excess calories when infection rates are reduced, if diets are not changed (Crimmins & Finch 2006). Some of the extra calories can be used for better growth and development; but then body weight may be increased.
Our results indicate that education and place of residence play an important role for understanding changes in health in the adult Mexican population. Mexicans with low education are significantly shorter in height and more likely to be overweight. For example, people with low education are about 4.5 times more likely to be stunted and both men and women with low education are more likely to be overweight. These results are consistent with previous research in which Mexican females with low education were significantly more likely to be obese (Beltrán-Sánchez, et al. 2011). It has also been suggested that obesity is more likely to occur later in life among those who were initially nutritionally deprived and then had more access to food (Hales & Barker 2001). There is also indication of differential health profiles being linked to timing of demographic and epidemiologic circumstances. People living in a state with a later epidemiological transition are more likely to be shorter or stunted (with odds of stunting twice as high), but rural dwelling males are less likely to be overweight as are females who live in poor States. These results suggest that total height and nutrition are associated with urbanization. This is consistent with previous studies in Mexico and in other low-income countries in which the percent urban is significantly associated with overweight and obesity (Beltrán-Sánchez, et al. 2011; Crimmins, et al. 2013; Sobngwi et al. 2004).
Finally, we also expected differences in the cardiovascular and metabolic profiles by age with a larger number of high-risk factors among the old. Our findings indicate that the rise in dysregulation is occurring at younger ages among Mexicans than Americans. We found a rapid increase with age in high plasma glucose for both men and women, from about 10% prevalence among people aged 20–29 to about 40% among those aged 70+, but marked differences by age and sex. The prevalence of high cholesterol and low HDL cholesterol was higher among women than men in Mexico, particularly at older ages. In a study of 4 high-income (England, Japan, U.S, and Taiwan) and 4 low-income countries (Bolivia, China, Indonesia, and Mexico), Crimmins and colleagues (2013) found a similar pattern in which females in low-income countries had much higher prevalence of low HDL cholesterol than males. In addition, high cholesterol in the Mexican population showed a similar prevalence across education groups for both males and females, but low HDL cholesterol and high plasma glucose levels were differentially linked to level of education. Low education is directly related to low HDL levels and inversely linked with high plasma glucose levels for both males and females. Females but not males living in a poor state are less likely to have worse HDL cholesterol levels consistent with previous research showing that people living in rural areas in Mexico have lower odds of being overweight and obese (Beltrán-Sánchez, et al. 2011). Similarly, a study in Sub-Saharan Africa showed that rural dwellers had lower BMI, lower glucose plasma levels, and lower blood pressure relative to urban residents (Njelekela et al. 2003; Sobngwi, et al. 2004). Finally, stunting is consistently linked to higher odds of worse lipid levels among males, but the opposite is true among females, while stunting increases the odds of having high plasma glucose. These results are consistent with the hypothesis that impaired physical growth associates with higher lipid levels and increased susceptibility to type-2 diabetes (Barker et al. 1993; Hales & Barker 2001).
Hypertension is quite high in Mexico at the younger adult ages, relative to the United States. Within Mexico, overweight is a strong predictor of more hypertension indicating that this pathway may be an important way that body weight is adversely affecting health. Interestingly, being stunted is linked to lower levels of hypertension. This is the opposite of what would be predicted from some of Barker’s work on early development and hypertension (Barker et al. 2002).
Our use of summary indicators helps to generalize some of the individual maker results. The overall higher level of physiological dysregulation among Mexicans compared to Americans results from dysregulation in multiple systems: higher levels of blood pressure, plasma glucose, and especially for women dysregulated cholesterol and higher body weight. The differing links by sex between education, rural residence, and State of residence indicate that the ongoing process of health change in Mexico may vary by gender in complex ways.
There are some limitations in our work. We have used our results to hint at the effects of the recent epidemiologic and nutrition transitions experienced in Mexico; but we have not been able to link these transitions directly to physiology. We assume that some of our sociodemographic indicators reflect life course exposures to these changes but this may not be fully captured in our cross-sectional data. We assume our results on age differences for some indicators determined in childhood reflect cohort differences but other physiological indicators are affected by both childhood and adulthood exposure and are less clearly reflective of cohort changes, as we cannot disentangle age and cohort effects. Finally, a portion of survey participants were excluded due to missing data. These exclusions were older and had low levels of education. For example, older people were more likely not to report their education, and among those who did report their schooling, older people and those with low education were more likely to have missing biomarker data. These characteristics are also related to some of the main results. For instance, older Mexicans are more likely to be stunted and underweight, and those with low education are more likely to be overweight and have worse lipid levels (high total cholesterol and low HDL cholesterol). Thus, by excluding individuals with missing data our results are likely to be conservative; had we been able to include them in the analyses, we might have seen stronger effects particularly for stunting and underweight.
On the other hand, the advantage of the current study is the use of clinically measured biomarkers of health. These indicators of physiological status and dysregulation provide a more comprehensive picture of population health as well as some hints of how health has changed over time in the adult Mexican population and how it differs from that in the U.S.
Conclusion
Our results indicate that increases in nutrition may have reached a point of diminishing returns as Mexico switched from a state of under-nutrition to over-nutrition. We show that differences in the health profile of the Mexican population by age, gender, education, and place of residence correspond to the salient demographic and socioeconomic characteristics of differential lifetime exposure to risk for physiological dysregulation. We provided a more comprehensive picture of population health as well as some hints of how the health profile has changed over time.
References
- Aguilar-Salinas CA, Gómez-Pérez FJ, Rull J, Villalpando S, Barquera S, Rojas R. Prevalence of dyslipidemias in the mexican national health and nutrition survey 2006. Salud Pública de México. 2010;52(Suppl 1):S44–S53. doi: 10.1590/s0036-36342010000700008. [DOI] [PubMed] [Google Scholar]
- Aguirre-Arenas J, Escobar-Pérez M, Chávez-Villasana A. Evaluación de los patrones alimentarios y la nutrición en cuatro comunidades rurales. Salud Pública de México. 1998;40(5):398–407. [PubMed] [Google Scholar]
- Aguirre A. Evolución de la mortalidad en américa latina, 1960–1990 y perspectivas de reducción de mortalidad infantil. In: Hill K, Morelos JB, Wong R, editors. Las consecuencias de las transiciones demográfica y epidemiológica an america latina. Mexico, D.F: El Colegio de Mexico; 1999. pp. 45–56. [Google Scholar]
- Barker DJP. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13(9):807–813. doi: 10.1016/s0899-9007(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Forsén T, Eriksson JG, Osmond C. Growth and living conditions in childhood and hypertension in adult life: A longitudinal study. Journal of Hypertension. 2002;20(10):1951–1956. doi: 10.1097/00004872-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Martyn CN, Osmond C, Hales CN, Fall CH. Growth in utero and serum cholesterol concentrations in adult life. British Medical Journal. 1993;307(6918):1524–1527. doi: 10.1136/bmj.307.6918.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barquera S, Campos-Nonato I, Carrión-Rábago C, Villalpando S, López-Ridaura R, Rojas R, Aguilar-Salinas CA. Methodology for the analysis of type 2 diabetes, metabolic syndrome and cardiovascular disease risk indicators in the ensanut 2006. Salud Pública de México. 2010;52(Suppl 1):S4–S10. doi: 10.1590/s0036-36342010000700003. [DOI] [PubMed] [Google Scholar]
- Barquera S, Campos-Nonato I, Hernández-Barrera L, Flores M, Durazo-Arvizu R, Kanter R, Rivera JA. Obesity and central adiposity in mexican adults: Results from the mexican national health and nutrition survey 2006. Salud Pública de México. 2009;51(Suppl 4):S595–S603. doi: 10.1590/s0036-36342009001000014. [DOI] [PubMed] [Google Scholar]
- Barquera S, Campos-Nonato I, Hernández-Barrera L, Villalpando S, Rodríguez-Gilabert C, Durazo-Arvizú R, Aguilar-Salinas CA. Hypertension in mexican adults: Results from the national health and nutrition survey 2006. Salud Pública de México. 2010;52(Suppl 1):S63–S71. doi: 10.1590/s0036-36342010000700010. [DOI] [PubMed] [Google Scholar]
- Barquera S, Hotz C, Rivera J, Tolentino L, Espinoza J, Campos I, Shamah T. Food and Agriculture Organization of the United Nations, editor. The double burden of malnutrition: Case studies from six developing countries. 2006. Food consumption, food expenditure, anthropometric status and nutrition-related diseases in mexico; pp. 161–203. [Google Scholar]
- Beltrán-Sánchez H, Crimmins EM, Teruel GM, Thomas D. Links between childhood and adult social circumstances and obesity and hypertension in the mexican population. Journal of Aging and Health. 2011;23(7):1141–1165. doi: 10.1177/0898264311422255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: Conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology. 2002;31(2):285–293. [PubMed] [Google Scholar]
- Camposortega Cruz S. Cambios en la mortalidad: Cien años de mortalidad en mexico [changes in mortality: A century of mortality in mexico] Demos. 1997;12:11–13. [Google Scholar]
- Centers for Disease Control and Prevention (CDC), & National Center for Health Statistics (NCHS) National health and nutrition examination survey protocol. 2012 Retrieved Oct 30, 2012, from http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm.
- CONAPO. infant mortality by locality, 2005Tasa de mortalidad infantil por municipio, 2005. 2012 Retrieved Oct 30, 2012, from http://www.conapo.gob.mx/en/CONAPO/Base_de_datos.
- Consejo Nacional de Evaluación de la Politica de Desarrollo Social. Los mapas de la pobreza en méxico. 2007 Retrieved Oct 30, 2012, from http://www.coneval.gob.mx/mapas/mapas/presentacion.pdf.
- Consejo Nacional de Población. La situacion demográfica de mexico 2010. [mexico’s demographic state 2010]. Mexico, D.F: Consejo Nacional de Población; 2010. [Google Scholar]
- Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(2):498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Kim JK, Seeman TE. Poverty and biological risk: The earlier “aging” of the poor. Journals of Gerontology - Series A Biological Sciences and Medical Sciences. 2009;64(2):286–292. doi: 10.1093/gerona/gln010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, Preston SH, Cohen B. Explaining divergent levels of longevity in high-income countries. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- Crimmins EM, Wheaton F, Vasunilashorn S, Beltrán-Sánchez H, Zhang L, Kim JK. A global perspective on physiological change with age. In: Mc-Donald SA, Zimmer Z, editors. Global ageing in the twenty-first century: Challenges, opportunities and implications. England: Ashgate Publishing Ltd; 2013. [Google Scholar]
- Finch CE. The biology of human longevity: Inflammation, nutrition, and aging in the evolution of life spans. Burlington, MA: Academic Press; 2007. [Google Scholar]
- Fogel RW, Costa DL. A theory of technophysio evolution, with some implications for forecasting population, health care costs, and pension costs. Demography. 1997;34(1):49–66. [PubMed] [Google Scholar]
- Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(38):14158–14163. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CN, Barker DJP. The thrifty phenotype hypothesis: Type 2 diabetes. British Medical Bulletin. 2001;60(1):5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Juarez-Rojas JG, Cardoso-Saldana GC, Posadas-Sanchez R, Medina-Urrutia AX, Yamamoto-Kimura L, Posadas-Romero C. Blood pressure and associated cardiovascular risk factors in adolescents of mexico city. Archivos de Cardiología de México. 2008;78(4):384–391. [PubMed] [Google Scholar]
- Lloyd-Jones DM, Wilson PW, Larson MG, Beiser A, Leip EP, D’Agostino RB, Levy D. Framingham risk score and prediction of lifetime risk for coronary heart disease. The American Journal of Cardiology. 2004;94(1):20–24. doi: 10.1016/j.amjcard.2004.03.023. [DOI] [PubMed] [Google Scholar]
- McKeown T, Record RG, Turner RD. An interpretation of the decline of mortality in england and wales during the twentieth century. Population Studies. 1975;29(3):391–422. [PubMed] [Google Scholar]
- Mitka M. Metabolic syndrome recasts old cardiac, diabetes risk factors as a “new” entity. JAMA. 2004;291(17):2062–2063. doi: 10.1001/jama.291.17.2062. [DOI] [PubMed] [Google Scholar]
- Montez JK, Hayward M. Early life conditions and later life mortality. In: Rogers R, Crimmins EM, editors. International handbook of adult mortality. Dordrecht: Springer; 2011. pp. 187–206. [Google Scholar]
- Njelekela M, Sato T, Nara Y, Miki T, Kuga S, Noguchi T, Kanda T, Yamori M, Ntogwisangu J, Masesa Z, Mashalla Y, Mtabaji J, Yamori Y. Nutritional variation and cardiovascular risk factors in tanzania--rural-urban difference. South African Medical Journal. 2003;93(4):295–299. [PubMed] [Google Scholar]
- Olaiz-Fernández G, Rivera-Dommarco J, Shamah-Levy T, Rojas R, Villalpando-Hernández S, Hernández-Avila M, Sepúlveda-Amor J. Encuesta nacional de salud y nutrición 2006. Cuernavaca, Morelos, México: Instituto Nacional de Salud Pública; 2006. [Google Scholar]
- Palloni A, Soldo BJ, Wong R, McEniry M. Working Paper Series. Center for Demography and Ecology, University of Madison-Wisconsin; 2003. Health status in a national sample of elderly mexicans. 2003-03. Retrieved from http://www.ssc.wisc.edu/cde/cdewp/2003-03.pdf. [Google Scholar]
- Palloni A, Wyrick R. Mortality decline in latin-america - changes in the structure of causes of deaths, 1950–1975. Social Biology. 1981;28(3–4):187–216. doi: 10.1080/19485565.1981.9988458. [DOI] [PubMed] [Google Scholar]
- Popkin BM. The shift in stages of the nutrition transition in the developing world differs from past experiences! Public Health Nutrition. 2002;5(1A):205–214. doi: 10.1079/PHN2001295. [DOI] [PubMed] [Google Scholar]
- Popkin BM. Global nutrition dynamics: The world is shifting rapidly toward a diet linked with noncommunicable diseases. American Journal of Clinical Nutrition. 2006;84(2):289–298. doi: 10.1093/ajcn/84.1.289. [DOI] [PubMed] [Google Scholar]
- Rivera JA, Barquera S, Campirano F, Campos I, Safdie M, Tovar V. Epidemiological and nutritional transition in mexico: Rapid increase of non-communicable chronic diseases and obesity. Public Health Nutrition. 2002;5(1A):113–122. doi: 10.1079/PHN2001282. [DOI] [PubMed] [Google Scholar]
- Rivera JA, Barquera S, Gonzalez-Cossio T, Olaiz G, Sepulveda J. Nutrition transition in mexico and in other latin american countries. Nutrition Reviews. 2004;62(II):S149–S157. doi: 10.1111/j.1753-4887.2004.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Samper-Ternent R, Michaels-Obregon A, Wong R, Palloni A. Older adults under a mixed regime of infectious and chronic diseases. Salud Pública de México. 2012;54(5):487–495. doi: 10.1590/s0036-36342012000500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: Macarthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(8):4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobngwi E, Mbanya JC, Unwin NC, Porcher R, Kengne AP, Fezeu L, Minkoulou EM, Tournoux C, Gautier JF, Aspray TJ, Alberti K. Exposure over the life course to an urban environment and its relation with obesity, diabetes, and hypertension in rural and urban cameroon. International Journal of Epidemiology. 2004;33(4):769–776. doi: 10.1093/ije/dyh044. [DOI] [PubMed] [Google Scholar]
- Warner DF, Hayward MD. Early-life origins of the race gap in men’s mortality. Journal of Health and Social Behavior. 2006;47(3):209–226. doi: 10.1177/002214650604700302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Espinoza M, Palloni A. Adultos mayores mexicanos en contexto socioeconómico amplio: Salud y envejecimiento. Salud Pública de México. 2007;49(Suppl 4):S436–S447. doi: 10.1590/s0036-36342007001000002. [DOI] [PubMed] [Google Scholar]
- World Health Organization. World health statistics 2012. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- Yamamoto-Kimura L, Posadas-Romero C, Posadas-Sanchez R, Zamora-Gonzalez J, Cardoso-Saldana G, Ramirez IM. Prevalence and interrelations of cardiovascular risk factors in urban and rural mexican adolescents. Journal of Adolescent Health. 2006;38(5):591–598. doi: 10.1016/j.jadohealth.2005.04.004. [DOI] [PubMed] [Google Scholar]