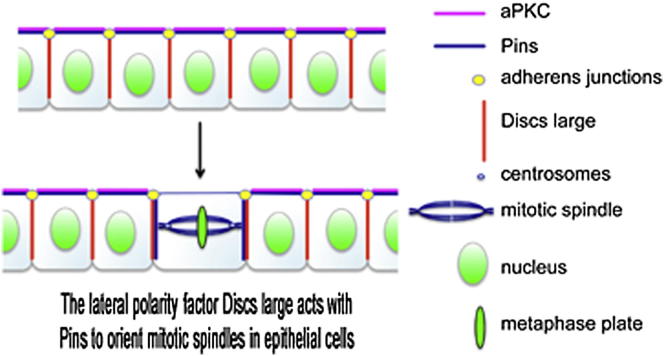
Summary
Mitotic spindles in epithelial cells are oriented in the plane of the epithelium so that both daughter cells remain within the monolayer, and defects in spindle orientation have been proposed to promote tumorigenesis by causing epithelial disorganization and hyperplasia [1]. Previous work has implicated the apical polarity factor aPKC, the junctional protein APC2, and basal integrins in epithelial spindle orientation, but the underlying mechanisms remain unclear. We show that these factors are not required for spindle orientation in the Drosophila follicular epithelium. Furthermore, aPKC and other apical polarity factors disappear from the apical membrane in mitosis. Instead, spindle orientation requires the lateral factor Discs large (Dlg), a function that is separable from its role in epithelial polarity. In neuroblasts, Pins recruits Dlg and Mud to form an apical complex that orients spindles along the apical-basal axis. We show that Pins and Mud are also necessary for spindle orientation in follicle cells, as is the interaction between Dlg and Pins. Dlg localizes independently of Pins, however, suggesting that its lateral localization determines the planar orientation of the spindle in epithelial cells. Thus, different mechanisms recruit the conserved Dlg/Pins/Mud complex to orient the spindle in opposite directions in distinct cell types.
Graphical Abstract
Highlights
-
•
Spindle orientation in follicle cells does not require aPKC, APC2, or integrins
-
•
aPKC and other apical polarity factors disappear from the cortex during mitosis
-
•
Dlg functions with Pins and Mud to orient the spindle toward the lateral cortex
-
•
Dlg localization does not require Pins and may determine spindle orientation
Results and Discussion
Mitotic Spindle Orientation in the Follicle Cell Epithelium Is Independent of Integrins and Adherens Junctions
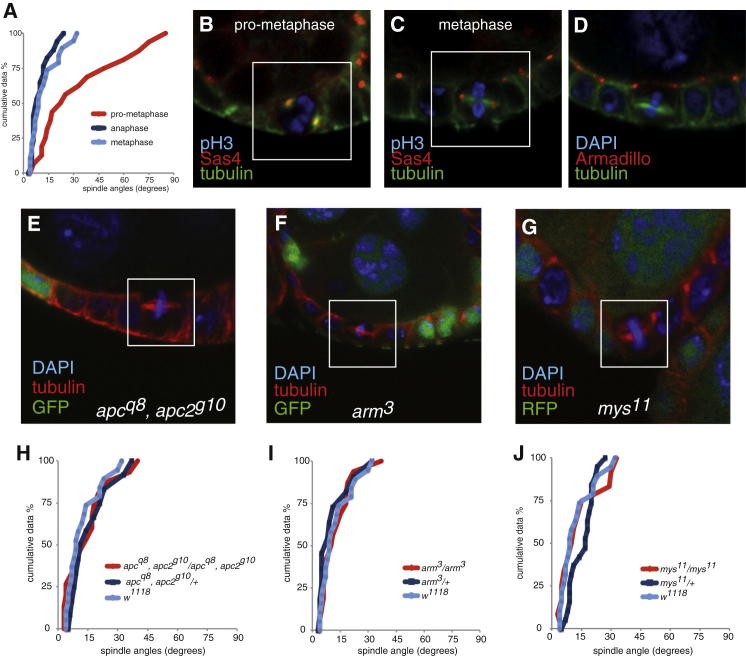
The Drosophila follicular epithelium is a well-established model for the study of epithelial cell polarity, but mitotic spindle orientation in this tissue has received less attention. Previous work has demonstrated that the follicle cells behave like a typical epithelium and tend to orient their mitotic spindles parallel to the plane of the monolayer [2]. Consistent with this, we observed that all spindles lie within ∼30° of the plane of the epithelium once the spindle and metaphase plate are clearly visible, and the spindle retains this orientation through anaphase (Figures 1A and 1C). The position of the spindle is much more variable earlier in mitosis, however, and the spindle often assembles perpendicular to the plane of the epithelium (Figures 1A and 1B and Figures S1A and S1B available online). Thus, the spindle is positioned after it has assembled and is not oriented by the prepositioning of the centrosomes.
Figure 1.
At Metaphase, Spindles Are Angled Roughly in Parallel to the Plane of the Follicle Cell Epithelium
(A) Cumulative plot of spindle angles in the FCE at prometaphase (red line), metaphase (blue), and anaphase (dark blue). The spindle angles were measured relative to a line through the adherens junctions, which represents the plane of the epithelium, and were plotted in rank order from lowest to highest against the spindle angles along the x axis.
(B and C) Mitotic spindles at prometaphase (B) and metaphase (C). Sas4-GFP (red) marks the spindle poles, and α-phospho-Histone 3 (pH3; blue) marks mitotic cells.
(D) Mitotic spindles (green) in follicle cells orient below the level of the adherens junctions, which are marked by Armadillo (Drosophila β-catenin) in red.
(E and F) Metaphase spindles are not misoriented in apc1q8, apc2g10 (E), or arm3 (F) homozygous mutant clones, marked by the absence of GFP (green). Metaphase cells are outlined by white boxes.
(G) Metaphase spindles are normally oriented in single-layer mys11 clones (marked by the absence of RFP in green).
(H–J) Cumulative plots of spindle angles in apc1q8, apc2g10 (H), arm3 (I), and mys11 (J) mutant follicle cells.
See also Figure S1.
The original model for spindle orientation in Drosophila epithelia based on studies in the embryonic ectoderm proposed that the spindles align toward the adherens junctions (AJ) through interactions mediated by APC2 (a homolog of mammalian adenenomatous polyposis coli) [3]. We observed that mitotic follicle cells maintain adherens junctions with their neighboring cells during metaphase (Figure 1D). However, the metaphase spindle is always positioned below the level of the AJ marker Armadillo (Drosophila β-catenin) (Figure 1D). APC-2-GFP is slightly enriched at adherens junctions in mitotic cells but also localizes around the rest of the cortex (Figure S1B). Furthermore, there is no significant change in spindle orientation in follicle cell clones that are doubly mutant for null alleles of apc1 and apc2 (Figures 1E and 1H), consistent with a previous report that APC2 is not required for spindle orientation in the embryonic ectoderm [4].
We further investigated the role of adherens junctions in spindle orientation by removing them completely using a null allele of arm. As reported previously, we saw some flattening and loss of epithelial cells in arm3 homozygous mutant clones [5], but spindle orientation was wild-type (Figure S1C and Figures 1F and 1I). Thus, adherens junctions do not play a role in mitotic spindle orientation in this tissue.
More recently, it has been suggested that integrin adhesion to the ECM is required for spindle orientation in epithelia. This suggestion is based on the observation that follicle cell clones mutant for the protein null allele myospheroid11 (βPS integrin), are characterized by both disorganized clusters of cells and misoriented mitotic spindles (Figures S1G and S1J) [2]. We never observed spindles oriented at >35° from the epithelial plane in mys11 mutant clones that are still part of the epithelial monolayer, however, and the distribution of spindle orientations within these clones was indistinguishable from that of the wild-type (Figures 1I and 1J). Spindle orientation is much more variable in the multilayered clusters of disorganized cells, but this is probably a secondary consequence of the loss of epithelial organization. Integrins are therefore dispensable for spindle orientation in the follicle cells and must disrupt epithelial organization by some other mechanism.
Spindle Orientation in Follicle Cells Does Not Require aPKC
The apical polarity factor atypical protein kinase C (aPKC) has been implicated in spindle orientation in Madin-Darby canine kidney (MDCK) cells and the Drosophila imaginal wing disc epithelium, although it does not appear to play a role in chick neuroepithelial cells [6–8]. We therefore analyzed the role of aPKC during mitosis in the follicular epithelium.
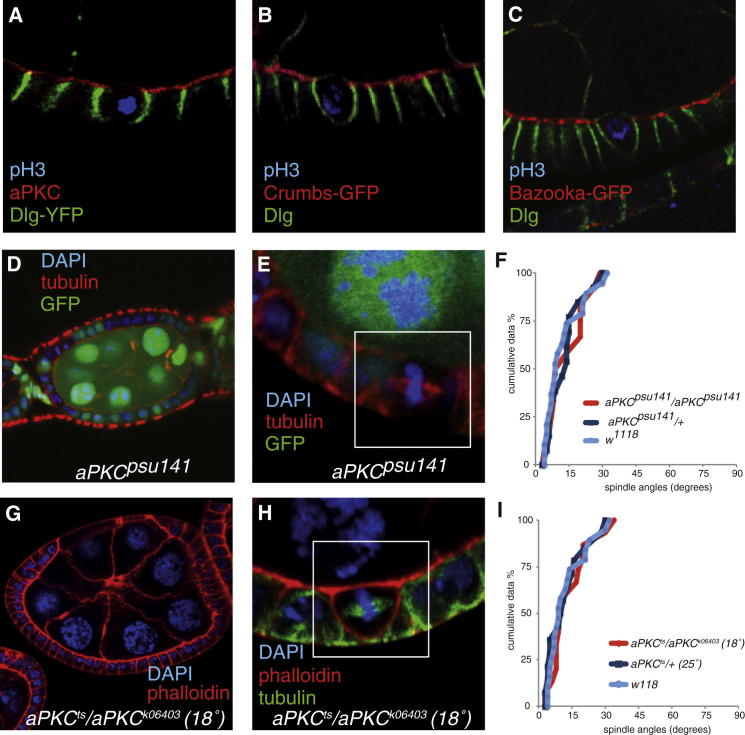
Surprisingly, we observed a loss of apical cortical identity during mitosis. aPKC disappears from the apical cortex, as do other apical polarity factors, such as Crumbs and Bazooka (Figures 2A–2C) [9]. Thus, aPKC is not in the right place to control the positioning of the spindle during metaphase. Consistent with this, follicle cells homozygous for the “kinase-dead” allele apkcpsu141 [10], show normal spindle orientation (Figures 2E and 2F). This allele does not disrupt the follicle epithelium (Figure 2D), suggesting that the kinase activity of aPKC is not required for epithelial polarity in this tissue.
Figure 2.
Spindle Orientation in Follicle Cells Does Not Depend on aPKC
(A–C) The apical polarity determinants aPKC (A, red), Crumbs-GFP (B, red), and Bazooka (C, red), disappear from the apical cortex of mitotic follicle cells, although Dlg (green) remains along the lateral cortex. Dlg-YFP is shown in (A), and anti-Dlg staining is shown in (B) and (C). pH3, shown in blue, marks mitotic cells.
(D) aPKCpsu14 mutant follicle cell clones have normal epithelial architecture. The aPKCpsu14 mutant cells are marked by the loss of GFP (green).
(E) Metaphase spindles are not misoriented in aPKCpsu14 mutant follicle cell clones (marked by the absence of GFP).
(F) Cumulative plot of metaphase spindle angles in aPKCpsu141 mutant follicle cells.
(G) An apkcts/apkck06403 egg chamber at 18° showing a cyst encapsulation defect, as well as normal tissue.
(H) Metaphase spindles are correctly oriented in an apkcts/apkck06403 egg chamber at 18°.
(I) Cumulative plot of metaphase spindle angles in apkcts/apkck06403 follicle cells at 18°.
Egg chambers transheterozygous for apkcts and the loss-of-function allele apkck06403, have cyst encapsulation defects at 25° that preclude the accurate measurement of mitotic spindle angles [8]. At 18°, large regions of organized epithelium persist in most egg chambers, although 68% (n = 28) show some encapsulation defects (Figure 2G). The follicle cells in the regions with normal epithelial organization show no spindle orientation defects (Figures 2H and 2I). The loss of apical aPKC in mitosis and the normal distribution of mitotic spindles in aPKC mutants indicate that aPKC is dispensable for spindle orientation in the follicular epithelium.
Pins and Mud Are Involved in Spindle Orientation
Much of our understanding of spindle orientation derives from studies of asymmetric divisions in the nematode and the fly (recently reviewed in [11, 12]). In Drosophila, spindle orientation has been studied primarily in neuroblasts, which divide asymmetrically along their apical-basal axis, and in the sensory organ precursor cell, which divides along a planar axis [12]. Additional studies have been carried out using a cultured Drosophila cell system with artificially induced cell polarity [13]. This work has identified a number of factors that are required to orient the spindle, including Partner of Inscuteable (Pins) and its binding partner Mushroom body defective (Mud) (reviewed in [12]). Work in symmetrically dividing vertebrate cells, including cultured MDCK cells, the chick neuroepithelium, and asymmetrically dividing skin cells has revealed that spindle orientation also depends on the Pins and Mud homologs LGN and NuMA [12, 14–18].
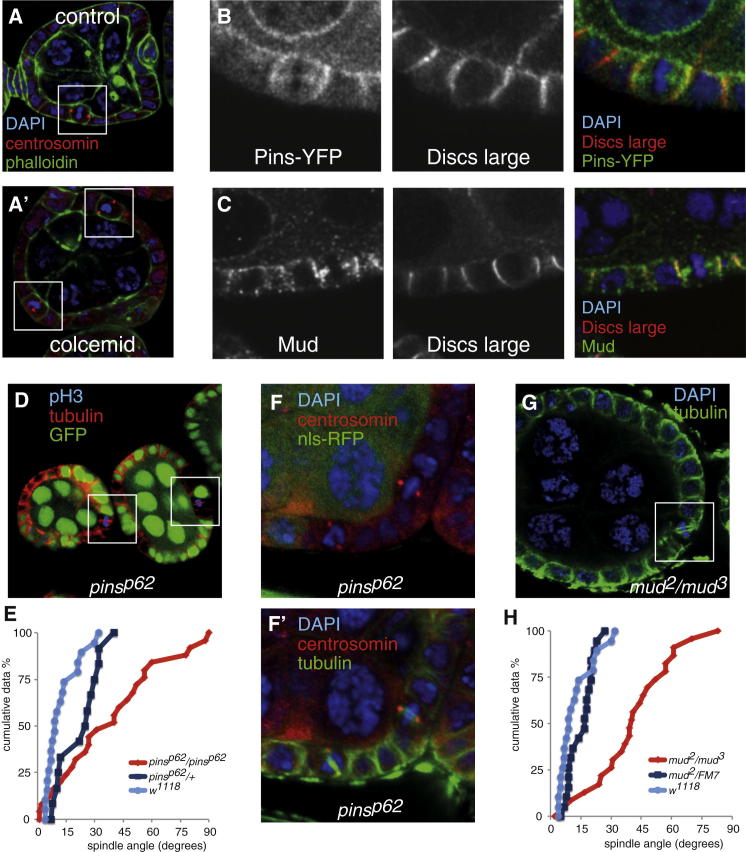
Pins and Mud orient the spindle by exerting a pulling force on astral microtubules through dynein/dynactin [19, 20]. If they are playing a role in spindle orientation in the follicular epithelium, then metaphase plates should misorient when astral microtubules are lost. We treated ovaries with the microtubule-depolymerizing drug colcemid for 1 hr and observed that metaphase plates and centrosomes were often misoriented with respect to the plane of the epithelium (Figures S2A and S2B and Figure 3A), indicating that microtubules are required for spindle orientation.
Figure 3.
Pins and Mud Are Required for Spindle Orientation in the Follicle Cells
(A and A′) The centrosomes (marked by centrosomin, red) align with the lateral cortex in untreated cells, and the metaphase plate (marked with DAPI, blue) is positioned vertically. The centrosomes and the metaphase plate are misoriented after treatment with the microtubule depolymerizing drug colcemid.
(B and C) Pins-YFP (B, red) and Mud (C, red) colocalize with Dlg (green) along the lateral cortex in mitotic follicle cells (DAPI, blue).
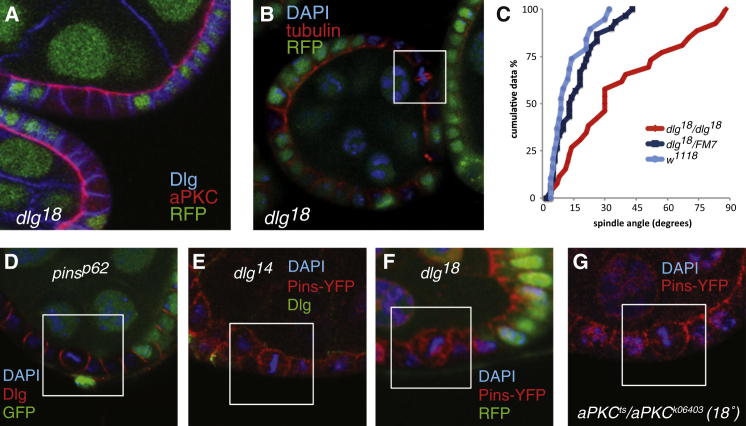
(D) Metaphase spindles are misoriented in the absence of pins function. Cells homozygous for pinsp62 are marked by the absence of GFP (green). Boxes highlight two metaphase cells with misaligned spindles.
(E) Cumulative plot of metaphase spindle angles in pinsp62 mutant cells showing that the spindles are randomly oriented. Spindle angles in pinsp62 mutant cells (n = 32) differ significantly from the wild-type, with a p value of <0.005 as determined by the Kolmogorov-Smirnov test.
(F and F′) pinsp62 mutant cells marked by the absence of RFP (green in F) form normal bipolar spindles (green in F′) with centrosomes at each pole (marked by Centrosomin, in red).
(G) Metaphase spindles are misoriented in mud2/mud3 follicle cells.
(H) Cumulative plot of metaphase spindle angles in mud2/mud3 mutant follicle cells showing spindle misorientation with clustering at approximately 45°. Spindle angles in mud2/mud3 mutant cells (n = 24) differ significantly from those in the wild-type (p value of <0.005 as determined by the Kolmogorov-Smirnov test). See also Figure S2.
We next examined the expression of Pins and Mud in follicle cells. In the adult fly, Pins transcript expression is highest in the ovary (Figure S2C). Immunostaining reveals that Mud is expressed in the follicle cell epithelium up until approximately stage 6 of egg chamber development, which is when the follicle cells cease dividing (Figure S2D). In the chick neuroepithelium and in other models, Pins (LGN) and Mud (NuMA) localize along the lateral cortex in dividing cells [12, 17]. Pins-YFP localizes along the apical cortex in interphase follicle cells, but it largely relocalizes to the lateral cortex during metaphase, where it colocalizes with the lateral polarity factor Dlg (Figure 3B and Figure S2E). In agreement with earlier work, it can also be found on the spindle (Figure 3B) [14]. Immunostaining for native Pins reveals the same pattern (Figure S2B). Mud also localizes at the lateral cortex with Dlg in mitotic cells, as well as at spindle poles (Figure 3C). To examine the role of Pins in spindle positioning, we generated homozygous mutant clones for the loss of function allele pinsp62 (Figure 3D) [21, 22]. In contrast to wild-type and heterozygous cells, metaphase spindles were found at all angles in mutant cells (Figure 3E). The cumulative plot of angular distributions in pinsp62 best fits a straight line of slope of 1.1 (R2 = 0.98), which is close to random (slope = 1), compared to slopes of 3.3 and 2.8 in wild-type and heterozygous cells.
In mammalian cells, knockdown of the Pins homolog LGN causes spindle disorganization [14]. These defects were not observed in pinsp62 clones, as revealed by staining for tubulin, the centrosomal protein Centrosomin (Figures 3F and 3F′), and the microtubule-nucleating factor γ-tubulin (Figure S2C).
Metaphase spindle angles were also examined in follicle cells from mud2/mud3 transheterozygous egg chambers (Figure 3G). In agreement with previous work, spindle orientation was disrupted in these cells (Figure 3H) [23]. We note that the spindle angles were not randomized, as in pins mutant cells, as the line of best fit has a slope of 1.5. These data are statistically consistent with a normal (Gaussian) distribution around a mean of 40°. This reflects the fact that many spindles orient toward an apical corner, as shown in Figure 3G. This result is consistent with a previous study in S2 cells that showed that Pins can attract a spindle pole in the absence of Mud but cannot center it through pulling [13]. We suggest that this mechanism is also at work in mud mutant follicle cells; the developing spindle may be caught at the edge of a Pins basolateral crescent in the absence of Mud function.
Dlg Is Required for Spindle Orientation in Follicle Cells
Dlg is recruited by Pins to the cortex of asymmetrically dividing cells, such as neuroblasts and SOPs, and is required to orient the spindle toward the Pins crescent [13, 24, 25]. Since Dlg colocalizes with Pins and Mud at the lateral cortex of the follicle cells (Figures 3D and 3E), we investigated whether it is also necessary for spindle orientation in this epithelium. Dlg is essential for apical-basal polarity in epithelia, however. This complicates the analysis of its role in spindle orientation, because cells homozygous mutant for a strong loss-of-function allele, dlg14 (also called dlgm52), round up and lose their epithelial organization (Figure S3A) [26]. We therefore restricted our analysis to those dlg14 mutant clones in which the cells remained in a monolayer and observed that the spindles are randomly oriented (Figures S3B and S3C).
Dlg interacts with Pins through its C-terminal guanylate kinase (GUK) domain, which is disrupted in cells homozygous for the mutant allele dlg18 (also called dlg1P20), a premature stop mutation that removes the last 43 amino acids of the protein [27, 28]. Importantly, dlg18 does not disrupt the lateral localization of Dlg, and apical-basal polarity is unaffected in early-stage mutant clones, which form a normal epithelial monolayer (Figure 4A). Despite this wild-type epithelial organization, dlg18 randomizes the orientation of the mitotic spindles to give a cumulative distribution with a slope of 1.1 (R2 = 0.93) (Figures 4B and 4C).
Figure 4.
Dlg Is Required for Mitotic Spindle Orientation
(A) dlg18 mutant follicle cell clones (marked by the absence of RFP in green) maintain normal epithelial polarity until stage 6 of oogenesis, as shown by the normal localization of aPKC (red) apically and Dlg (blue) laterally.
(B) Metaphase spindles are misoriented in dlg18 mutant follicle cells.
(C) A cumulative plot of metaphase spindle angles in dlg18 mutant follicle cells. Spindle angles in dlg18 mutant cells (n = 27) differ significantly from those in the wild-type (p value of <0.005).
(D) Dlg localization is normal in mitotic pinsp62 cells. Mutant cells are marked by the absence of GFP (in green). Dlg (red) localizes to the lateral cortex in a mitotic cell, which is outlined by a white box. DAPI is in blue.
(E and F) Pins localizes to the apical and lateral cortex in dlg14 (E) and dlg18 (F) mutant cells. Mutant cells are marked by the absence of Dlg immunoreactivity (green in E) or RFP (green in F); Pins-YFP is shown in red.
(G) Pins-YFP (in red) localizes normally during mitosis in apkcts/apkck06403 at 18°.
See also Figure S3.
Spindles are oriented normally in dlgsw, which removes the last 14 amino acids of Dlg, leaving the GUK domain intact (Figures S3D–S3F) [24]. Thus, Dlg is required for spindle orientation in the follicle cells, and this function is separable from its role in epithelial polarity. The role of Dlg in spindle orientation depends on the presence of an intact GUK domain and therefore presumably requires its interaction with Pins, strongly suggesting that the Dlg/Pins/Mud complex orients the spindle in epithelia, as it does in asymmetrically dividing cells.
In neuroblasts, Pins is required for the apical localization of Dlg during mitosis, whereas Dlg reinforces the apical localization of Pins through a pathway that depends on astral microtubules [25]. The situation in epithelia appears to be different, however, as Dlg localizes normally along the lateral cortex in clones of the pins null mutant, pinsp62 (Figure 4D). Since Dlg localizes laterally throughout the cell cycle, it is presumably localized by the same polarity-related mechanisms in interphase and mitotic cells. We also examined whether Dlg is required for the localization of Pins and observed that Pins still localizes around the cortex during mitosis in the absence of Dlg (dlg14) but is not enriched laterally (Figure 4E). The lateral enrichment of Pins also appears reduced in cells homozygous for the GUK domain mutant dlg18, suggesting that its interaction with Dlg contributes to its recruitment to the lateral cortex, although this phenotype is more variable than in the null (Figure 4F).
It has previously been proposed that the aPKC excludes Pins from the apical domain during mitosis in MDCK cells and the Drosophila wing imaginal disc, although not in chick neuroepithelial cells [7, 8, 17]. In agreement with the latter finding, Pins-YFP shows a wild-type lateral localization during mitosis in apkcts/apkck06403 transheterozygous flies maintained at 18° (Figure 4G). Thus, the lateral enrichment of Pins in mitotic follicle cells is independent of aPKC.
In conclusion, we have demonstrated that the planar orientation of the mitotic spindle in the follicular epithelium is independent of apical, junctional, or basal cues and depends instead on Dlg, Pins, and Mud. It therefore seems likely that the spindle is aligned within the plane of the epithelium by the same mechanisms that orient the spindle along the apical-basal axis in neuroblasts and that the key determinant of spindle orientation in both cell types is the location of the Dlg/Pins/Mud complex. The restriction of this complex to the lateral cortex in epithelial cells depends on Dlg, and its dual role in apical-basal polarity and spindle positioning therefore provides a mechanism to couple spindle orientation with the overall polarity of the tissue.
Experimental Procedures
Drug treatment
Ovaries were suspended in Schneider’s medium (Sigma) containing 5 μg/ml insulin (Sigma) with or without 100 μg/ml colcemid (Sigma) for 1 hr before fixation.
Imaging
Somatic clone induction, immunofluorescence, and fixed-cell imaging were performed as previously described [29].
Spindle Angle Measurements
Spindle angles were calculated with Image J. The angle of the spindle was determined relative to a line drawn connecting the adherens junctions at the two apical corners of the mitotic cell. These corners are shared by this cell and its neighbors.
Acknowledgments
We thank Jordan Raff, Howard Nash, Chris Doe, Yohanns Bellaïche, Andreas Wodarz, and Jürgen Knoblich, and their labs for fly stocks. We are grateful to Eurico de Sa and other members of the St Johnston lab, the Piddini lab, and The Gurdon Institute for helpful criticism and comments made over the course of this study. This work was supported by a Wellcome Trust Principal Fellowship to D.S.J. (049818 and 080007) and core funding from the Wellcome Trust (092096) and Cancer Research UK (A14492). D.T.B. was supported by a Marie Curie Fellowship. H.E.L. was supported by a Herchel Smith Studentship.
Published: July 25, 2013
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Supplemental Information includes Supplemental Experimental Procedures and three figures and can be found with this article online at http://dx.doi.org/10.1016/j.cub.2013.07.017.
Supplemental Information
References
- 1.McCaffrey L.M., Macara I.G. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 2011;21:727–735. doi: 10.1016/j.tcb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Fernández-Miñán A., Martín-Bermudo M.D., González-Reyes A. Integrin signaling regulates spindle orientation in Drosophila to preserve the follicular-epithelium monolayer. Curr. Biol. 2007;17:683–688. doi: 10.1016/j.cub.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 3.Lu B., Roegiers F., Jan L.Y., Jan Y.N. Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature. 2001;409:522–525. doi: 10.1038/35054077. [DOI] [PubMed] [Google Scholar]
- 4.McCartney B.M., Price M.H., Webb R.L., Hayden M.A., Holot L.M., Zhou M., Bejsovec A., Peifer M. Testing hypotheses for the functions of APC family proteins using null and truncation alleles in Drosophila. Development. 2006;133:2407–2418. doi: 10.1242/dev.02398. [DOI] [PubMed] [Google Scholar]
- 5.Tanentzapf G., Smith C., McGlade J., Tepass U. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J. Cell Biol. 2000;151:891–904. doi: 10.1083/jcb.151.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox D.N., Seyfried S.A., Jan L.Y., Jan Y.N. Bazooka and atypical protein kinase C are required to regulate oocyte differentiation in the Drosophila ovary. Proc. Natl. Acad. Sci. USA. 2001;98:14475–14480. doi: 10.1073/pnas.261565198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao Y., Du Q., Chen X., Zheng Z., Balsbaugh J.L., Maitra S., Shabanowitz J., Hunt D.F., Macara I.G. Par3 controls epithelial spindle orientation by aPKC-mediated phosphorylation of apical Pins. Curr. Biol. 2010;20:1809–1818. doi: 10.1016/j.cub.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guilgur L.G., Prudêncio P., Ferreira T., Pimenta-Marques A.R., Martinho R.G. Drosophila aPKC is required for mitotic spindle orientation during symmetric division of epithelial cells. Development. 2012;139:503–513. doi: 10.1242/dev.071027. [DOI] [PubMed] [Google Scholar]
- 9.St Johnston D., Ahringer J. Cell polarity in eggs and epithelia: parallels and diversity. Cell. 2010;141:757–774. doi: 10.1016/j.cell.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Kim S., Gailite I., Moussian B., Luschnig S., Goette M., Fricke K., Honemann-Capito M., Grubmüller H., Wodarz A. Kinase-activity-independent functions of atypical protein kinase C in Drosophila. J. Cell Sci. 2009;122:3759–3771. doi: 10.1242/jcs.052514. [DOI] [PubMed] [Google Scholar]
- 11.Siller K.H., Doe C.Q. Spindle orientation during asymmetric cell division. Nat. Cell Biol. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- 12.Morin X., Bellaïche Y. Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Dev. Cell. 2011;21:102–119. doi: 10.1016/j.devcel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 13.Johnston C.A., Hirono K., Prehoda K.E., Doe C.Q. Identification of an Aurora-A/PinsLINKER/Dlg spindle orientation pathway using induced cell polarity in S2 cells. Cell. 2009;138:1150–1163. doi: 10.1016/j.cell.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du Q., Stukenberg P.T., Macara I.G. A mammalian Partner of inscuteable binds NuMA and regulates mitotic spindle organization. Nat. Cell Biol. 2001;3:1069–1075. doi: 10.1038/ncb1201-1069. [DOI] [PubMed] [Google Scholar]
- 15.Kaushik R., Yu F., Chia W., Yang X., Bahri S. Subcellular localization of LGN during mitosis: evidence for its cortical localization in mitotic cell culture systems and its requirement for normal cell cycle progression. Mol. Biol. Cell. 2003;14:3144–3155. doi: 10.1091/mbc.E03-04-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng Z., Zhu H., Wan Q., Liu J., Xiao Z., Siderovski D.P., Du Q. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J. Cell Biol. 2010;189:275–288. doi: 10.1083/jcb.200910021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peyre E., Jaouen F., Saadaoui M., Haren L., Merdes A., Durbec P., Morin X. A lateral belt of cortical LGN and NuMA guides mitotic spindle movements and planar division in neuroepithelial cells. J. Cell Biol. 2011;193:141–154. doi: 10.1083/jcb.201101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams S.E., Beronja S., Pasolli H.A., Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pecreaux J., Röper J.-C., Kruse K., Jülicher F., Hyman A.A., Grill S.W., Howard J. Spindle oscillations during asymmetric cell division require a threshold number of active cortical force generators. Curr. Biol. 2006;16:2111–2122. doi: 10.1016/j.cub.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Kotak S., Busso C., Gönczy P. Cortical dynein is critical for proper spindle positioning in human cells. J. Cell Biol. 2012;199:97–110. doi: 10.1083/jcb.201203166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu F., Morin X., Cai Y., Yang X., Chia W. Analysis of partner of inscuteable, a novel player of Drosophila asymmetric divisions, reveals two distinct steps in inscuteable apical localization. Cell. 2000;100:399–409. doi: 10.1016/s0092-8674(00)80676-5. [DOI] [PubMed] [Google Scholar]
- 22.Le Borgne R., Schweisguth F. Unequal segregation of Neuralized biases Notch activation during asymmetric cell division. Dev. Cell. 2003;5:139–148. doi: 10.1016/s1534-5807(03)00187-4. [DOI] [PubMed] [Google Scholar]
- 23.Yu J.X., Guan Z., Nash H.A. The mushroom body defect gene product is an essential component of the meiosis II spindle apparatus in Drosophila oocytes. Genetics. 2006;173:243–253. doi: 10.1534/genetics.105.051557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellaïche Y., Radovic A., Woods D.F., Hough C.D., Parmentier M.L., O’Kane C.J., Bryant P.J., Schweisguth F. The Partner of Inscuteable/Discs-large complex is required to establish planar polarity during asymmetric cell division in Drosophila. Cell. 2001;106:355–366. doi: 10.1016/s0092-8674(01)00444-5. [DOI] [PubMed] [Google Scholar]
- 25.Siegrist S.E., Doe C.Q. Microtubule-induced Pins/Galphai cortical polarity in Drosophila neuroblasts. Cell. 2005;123:1323–1335. doi: 10.1016/j.cell.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 26.Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- 27.Woods D.F., Hough C., Peel D., Callaini G., Bryant P.J. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J. Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston C.A., Doe C.Q., Prehoda K.E. Structure of an enzyme-derived phosphoprotein recognition domain. PLoS ONE. 2012;7:e36014. doi: 10.1371/journal.pone.0036014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morais-de-Sá E., Mirouse V., St Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010;141:509–523. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.