Fig. 2.
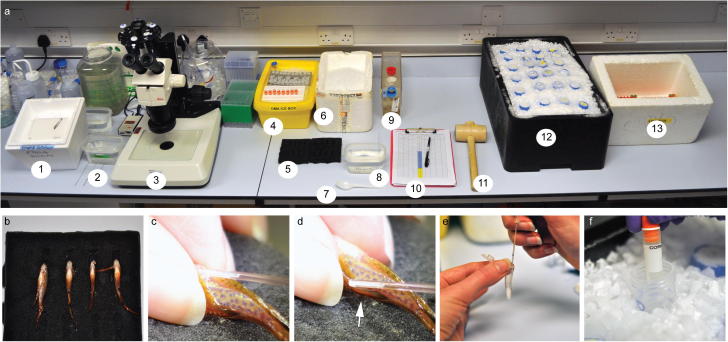
Cryopreservation of zebrafish sperm. From left to right the cryopreservation station comprises (1) dry ice block with deep well block for F1 tissue samples, (2) tricaine for culling, (3) LM80 dissecting microscope fitted with ring light, 1000 and 200 μl pipettes, bin and pipette tips behind, (4) wet ice box with tube rack for cryoprotectant aliquots and cryovials, (5) sponge with slits to hold anaesthetised fish, (6) wet ice box for cryovial box, (7) plastic spoon to scoop fish out of tricaine, (8) tricaine for anaesthesia, (9) fish tank holding males, (10) recording sheet, (11) mallet to drive Falcon tubes into dry ice, (12) dry ice/ethanol box with Falcon tubes, (13) styrofoam box containing 3 cm of LN2 and cryoboxes. Not depicted are: timer, tissues and cotton buds to dry off fish, suction tube and capillaries. (a) Males are placed in sponge holder ventral side up. (b) Carefully dried males are squeezed and sperm is collected in 10 μl capillary. (c) Good quality sperm is visible inside the capillary (white arrow). (d) Sperm is expelled into cryoprotectant while holding tube such that the solution is not warmed between the fingers. (e) Aliquots are pipetted into cryovials and immediately dropped into Falcon tubes which are capped and then hammered into the dry ice (f).