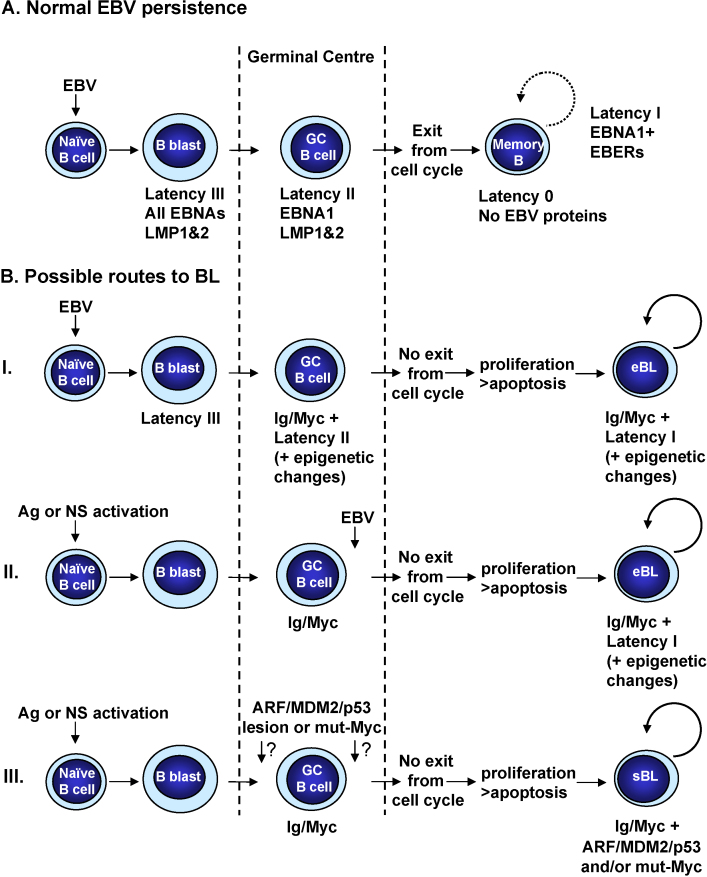
Fig. 2.
(A) EBV persistence. In vivo, EBV is thought to utilize normal B cell development and differentiation pathways to gain access to its site of long-term persistence, the memory B cell. The schematic is based on the model proposed by Thorley-Lawson and colleagues and is described more fully in the text. (B) Models of BL pathogenesis. (I) A naïve B cell infected with EBV expresses the latency III programme and is driven to proliferate. EBNA3A and EBNA3C are expressed and this can lead to the epigenetic repression of Bim. EBNA2 is down-regulated as the cells to differentiate into germinal centre (GC) cells. They now express only EBNA1, the LMPs and EBER RNAs but transcription of Bim is now significantly impaired. Ig/Myc translocation occurs during somatic hypermutation (SHM) and leads to uncontrolled proliferation. The deregulated Myc is potentially lethal, however, the prior repression of Bim transcription (and perhaps similar repression of p14ARF and p16INK4a) allows the cell to survive and proliferate. LMP1 and LMP2A may also repress apoptosis via NF-κB, Ras/PI3K/AKT and ERK/MAPK pathways and EBNA1 may prevent Myc-induced accumulation of p53. Myc-driven proliferation prevents the latently infected cell from becoming a resting memory cell and since the progeny remain in the division cycle, EBNA1 is expressed. The balance between proliferation and apoptosis will be maintained by the epigenetic repression of Bim (and perhaps other genes) together with the anti-apoptotic activities of EBNA1. (II) It is possible that during the pathogenesis of BL EBV might infect and rescue a GC B cell that has already sustained an Ig/Myc translocation. Such a cell may have entered a GC as the result of antigen (Ag) stimulation or some form of non-specific (NS) polyclonal activation as is common in malaria or HIV infections. Since latency III is the likely outcome of EBV infection, multiple survival factors will allow this B-blast-like cell to tolerate deregulation of Myc, but precisely how it exits from a germinal centre and how switching to latency I would occur is presently uncertain. Although the sequence of events differs from scenario (I), a latency III-expressing blast will again be the progenitor of BL. (III) In the absence of EBV, activated naïve B cells responding to a specific antigen (Ag) or non-specific (NS) polyclonal activation enter a germinal centre wherein AID is activated to initiate SHM and CRS. Very rarely an Ig/Myc translocation occurs as the result of aberrant CRS so the apoptosis threshold in this cell and its progeny will be dramatically reduced. For survival the primary targets seem to be p14ARF/MDM2/p53 and Myc/Bim, with a strong natural selection for mutations of p53 and/or codon 57/58 mutations of Myc (mut-Myc). See text for details and references.