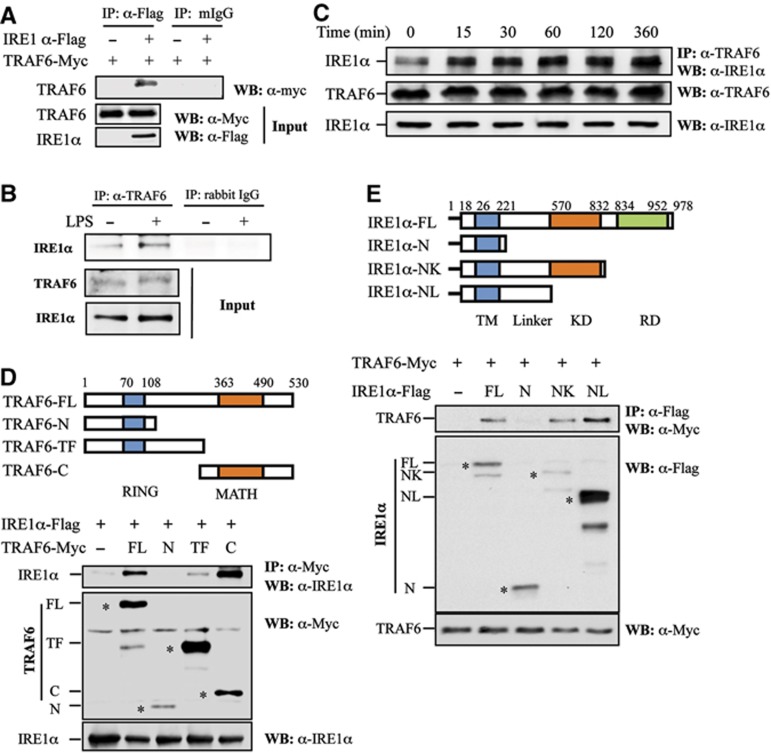
Figure 5.
TRAF6 interacts with IRE1α. (A) Flag-tagged IRE1α and Myc-tagged TRAF6 plasmids were co-transfected into HEK293 cells. IRE1α protein in the lysates of transfected cells was immunoprecipitated with an anti-Flag antibody or with normal mouse IgG (mIgG) as a control. The bound TRAF6 was determined by western blotting using an anti-Myc antibody (top panel). The expression levels of TRAF6 and IRE1α in whole cell lysates were confirmed by western blot analysis using anti-Myc (middle panel) and anti-Flag (bottom panel) antibodies, respectively. (B) Mouse primary macrophages derived from bone marrow cells were stimulated with or without LPS (1 g/ml) for 16 h. The interaction between TRAF6 and IRE1α was analysed by western blotting. (C) The interaction between TRAF6 and IRE1α in RAW cells stimulated with LPS (1 g/ml) under a time course was determined by co-immunoprecipitation and western blot analysis. (D) Schematic representation of TRAF6 and its truncated mutants. TRAF6 carries an N-terminal RING finger domain and a C-terminal MATH domain (top panel). FL: full-length structure, N: N-terminal RING finger domain, TF: trans-membrane domain, C: C-terminal MATH domain. IRE1α was co-transfected with TRAF6 or its mutants into HEK293 cells. The interactions between IRE1 and TRAF6 or its mutants were determined as described in (A). The expression of the full-length and truncated TRAF6 protein was indicated by the symbol ‘*’. (E) Schematic representation of IRE1α and its truncated mutants. IRE1α contains an N-terminal trans-membrane (TM) domain, kinase domain (KD), and a C-terminal RNase domain (RD) (top panel). FL: full length, N: N-terminal trans-membrane domain, NK: N-terminus and the kinase domain, NL: N-terminal linker domain. TRAF6 was co-transfected with IRE1α or its mutants into HEK293 cells. The interactions between TRAF6 and IRE1α or its mutants were determined as described in (A). The expression of the full-length and truncated IRE1α protein was indicated by the symbol ‘*’.
Source data for this figure is available on the online supplementary information page.