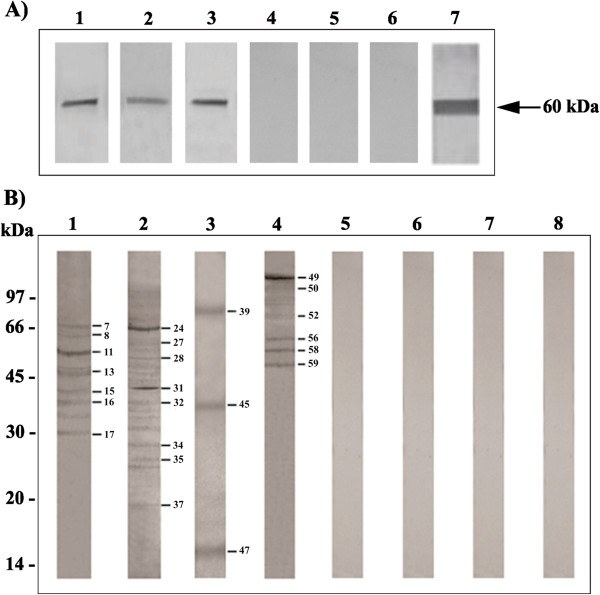
Figure 2.
Confirmation of the interactions by Far-Western blot assays. (A) PbMLS was subjected to SDS-PAGE and electro blotted. Membranes were reacted with Paracoccidioides protein extracts of mycelium (lane 1), yeast (lane 2) and macrophage (lane 3) and were subsequently incubated with anti-rabbit IgG anti-enolase, anti-triosephosphate isomerase and anti-actin, respectively. The reactions were revealed with anti-rabbit IgG conjugated to alkaline phosphatase. Negative control was obtained by incubating PbMLS with the antibodies anti-enolase, anti-triosephosphate isomerase and anti-actin, respectively, without preincubation with the protein extracts (lanes 4, 5 and 6). The positive control was obtained by incubating the PbMLS with the polyclonal anti-PbMLS antibody (lane 7). (B) Protein extracts of Paracoccidioides mycelium, yeast, secretions and macrophages (lanes 1, 2, 3 and 4, respectively) were subjected to SDS-PAGE and blotted onto nylon membrane. The membranes were incubated with PbMLS and, subsequently, primary antibody anti-PbMLS and secondary antibody anti-rabbit IgG. Negative control was obtained by incubating each protein extract with anti-PbMLS antibody, without preincubation with PbMLS (lanes 5, 6, 7 and 8). The numbers indicate the proteins (Additional file 2: Table S1) that interact with PbMLS that are confirmed by this technique.