Figure 2.
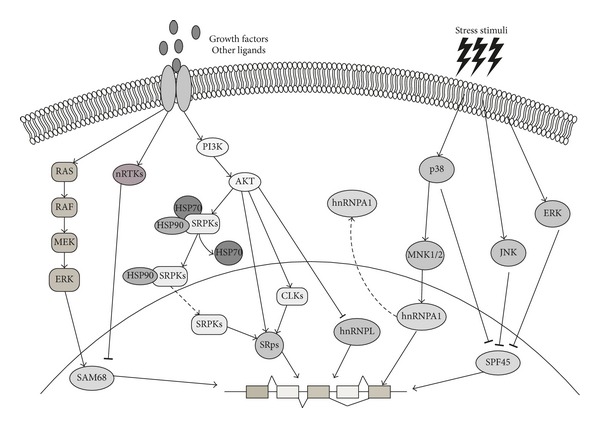
Signaling-activated kinases regulate splicing factor activity. Various extracellular cues, like growth factors or stress stimuli, activate different signal-transduction cascades impinging on protein kinases that in turn phosphorylate RBPs, thereby modulating their splicing activity. SAM68 splicing activity is inversely regulated by ERKs and nRTKs, which, respectively, activate and inhibit its splicing activity. The PI3 K-AKT pathway regulates the activity of several SR proteins both directly or by phosphorylating and modulating the activity and localization of CLKs and SRPKs. Stress signal-activated kinases, like JNK or p38, can both modulate splicing factor localization, like for hnRNPA1, or activity, like for SPF45 (see text for details).
