Abstract
More than 50 years of research has yielded numerous Shigella vaccine candidates that have exemplified both the promise of vaccine-induced prevention of shigellosis and the impediments to developing a safe and effective vaccine for widespread use, a goal that has yet to be attained. This Review discusses the most advanced strategies for Shigella vaccine development, the immune responses that are elicited following disease or vaccination, the factors that have accelerated or impeded Shigella vaccine development and our ideas for the way forward.
At the end of the 19th century, as epidemics of bacillary dysentery accompanied by high mortality spread across Japan, the young microbiologist Kiyoshi Shiga examined dysenteric stools and isolated a bacterium that was agglutinated by serum from convalescent patients but not from patients with acute disease1–3 (FIG. 1). That bacterium — known today as Shigella dysenteriae 1 — was the first identified member of the genus Shigella. Four Shigella species (or groups) are now recognized: S. dysenteriae (group A), which has 15 serotypes; Shigella flexneri (group B), which has 14 classical serotypes and subserotypes; Shigella boydii (group C), which has 20 serotypes; and Shigella sonnei (group D), which has single serotype4 (TABLE 1).
Figure 1.
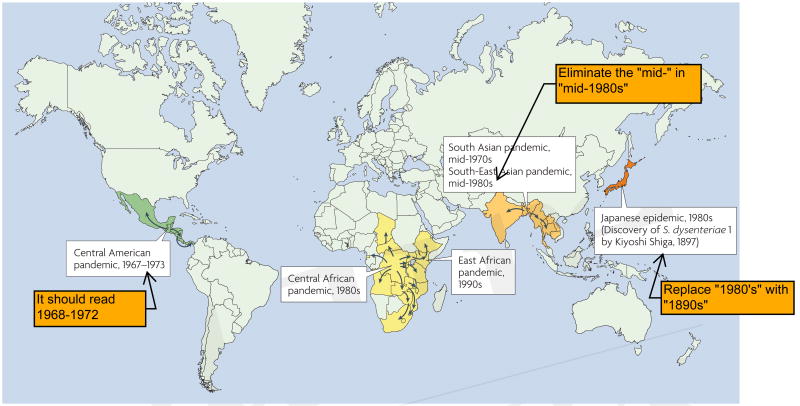
Pandemics of Shiga dysentery. The first member of the genus Shigella to be isolated, Shigella dysenteriae 1, was isolated by Kiyoshi Shiga during epidemics that occurred in Japan in the 1890s. Beginning in Central America in the late 1960s, pandemics of S. dysenteriae 1 caused severe disease with many complications and high case-fatality rates in all age groups. Subsequent pandemics occurred in Asia and Africa over the following 3 decades.
Table 1. Shigella species and serotypes.
| Shigella species (group) | Number of serotypes and subserotypes | Most important epidemiological niche | Serotypes that a global vaccine must protect against |
|---|---|---|---|
| S. dysenteriae (group A) | 15 | Epidemics and pandemics of severe Shiga dysentery | S. dysenteriae 1 |
| S. flexneri (group B) | 14* | Endemic paediatric shigellosis in developing countries | All 14 types |
| S. boydii (group C) | 20 | Scattered endemic foci in developing countries | None |
| S. sonnei (group D) | 1 | Travellers' diarrhoea; endemic shigellosis and outbreaks in developed and transitional countries | S. sonnei |
There are 14 classical Shigella flexneri serotypes and subserotypes4.
There has been resurgent interest in Shigella as a human pathogen, driven by the availability of more precise data on the disease burden5–8, emerging antibiotic resistance9,10 and the fact that mucosally invasive Shigella, which often cause dysentery (gross blood in diarrhoeal stools), are less amenable to the salutary effects of oral rehydration than non-invasive pathogens that cause watery diarrhoea, such as Vibrio cholerae and enterotoxigenic Escherichia coli. The target populations for the use of Shigella vaccines include infants and young children in developing countries (in which the peak incidence occurs at 12–47 months of age and the S. flexneri serotypes predominate)5–7.
S. dysenteriae 1, which produces Shiga toxin and typically carries R factors that encode resistance to multiple antibodies, waxes and wanes as a cause of epidemic severe disease in the world's least developed countries11– 14. Pandemics of Shiga dysentery, as occurred in Central America from ∼1967–1972 (REF. 11), South Asia in the 1970s12, Central Africa in the 1980s13 and East Africa in the 1990s14,15 (FIG. 1), profoundly influence the global mortality burden that can be attributed to Shigella5,15. The lack of S. dysenteriae 1 endemicity results in low background immunity in populations, so epidemics of Shiga dysentery affect adults and children alike and the target ages for the use of a Shiga vaccine would be similarly broad1,11–13.
S. sonnei persists in developed (and transitional) countries, causing sporadic diarrhoea and occasional outbreaks in epidemiological niches (such as day-care centres) where personal hygiene can be suboptimal16,17. Travellers from developed to developing regions, who mainly acquire S. sonnei and S. flexneri infections18, represent another target population for Shigella vaccines. Shigellosis due to S. boydii or S. dysenteriae serotypes other than type 1 is uncommon5–7 (although increases in S. boydii have been reported in some foci7). Consequently, a Shigella vaccine that can provide a high level of protection against S. dysenteriae 1, all S. flexneri serotypes and S. sonnei would constitute an epidemiologically valid ‘global’ vaccine5,19 (TABLE 1; BOX 1; FIG. 2).
Box 1. Shigella O-antigens.
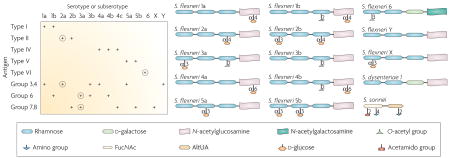
The lipopolysaccharide (LPS) of Shigella, a virulence factor119,120, consists of a toxic lipid A moiety embedded in the bacterial outer membrane, a core sugar region and an exposed terminal O polysaccharide. Whereas there is little diversity in lipid A and core regions among Shigella, the terminal O polysaccharide structures vary greatly, thereby giving rise to the immunological specificity that results in distinct serotypes (and subserotypes)4. Serotyping of Shigella flexneri is complicated. Current taxonomy recognizes 14 classical S. flexneri serotypes or subserotypes4, defined by type (I, II, IV–VI) and group (3, 4; 6;7, 8) antigens (see left panel of the figure). Notably, the group antigens are shared among different serotypes. Type III antigen is not shown in the figure, as no structure can be assigned to it118. Moreover, absorbed rabbit ‘type III’ antisera actually bind group 6 antigen, indicating that type III antigen is identical to group 6 antigen, which is present in other serotypes118. Nevertheless, re-classification is unnecessary as strains heretofore characterized as S. flexneri 3a and 3b can be readily identified with absorbed or monoclonal typing sera4,118,121; S. flexneri 3a agglutinate with antisera to group 6 and 7,8 antigens and S. flexneri 3b agglutinate with antisera to group 6 and 3,4 antigens (left panel)118.
Thirteen of the 14 classical S. flexneri serotypes or subserotypes (all except S. flexneri 6) share a common backbone of tetrasaccharide repeats that contain three rhamnose residues and one N-acetylglucosamine (right panel). Genes that encode proteins involved in the synthesis of the linear backbone are located in the rfb locus of the chromosome, whereas lysogenic phages encode the enzymes that link O-acetyl groups or d-glucose at various sites on the tetrasaccharide backbone, thereby creating or ablating antigenic epitopes. The resultant O-antigens, the structures of which are displayed schematically in the lower panel of the figure, provide the basis for the distinct S. flexneri serotypes or subserotypes. S. flexneri 6 has d-galactose as the third sugar of the tetrasaccharide and N-acetylgalactosamine as the terminal residue (right panel). The structure of S. flexneri 4c is not shown as it has not been reported.
As Shigella dysenteriae 1 and Shigella sonnei are two serotypes that should be included in a global Shigella vaccine, the basic structures of their O polysaccharide repeats are also shown103,122. In contrast to the other O-antigens that have tetrasaccharide repeats, the O-antigen repeat of S. sonnei is a disaccharide (FucNAc, 2-acetamido-4-amino-2,4,6-trideoxy-d-galactose; AltUA, 2-amino-2-deoxy-l-altruronic acid)103. The S. dysenteriae 1 O polysaccharide repeat most closely resembles the structures of S. flexneri 6 and S. flexneri Y of the S. flexneri subtypes.
The left panel displays the 14 S. flexneri serotypes and subserotypes along the horizontal axis and the type and group antigens in the vertical axis. Below each serotype or subserotype, the specific type and group antigens expressed are indicated by a plus sign. As the O-antigens (circled) of S. flexneri 2a and S. flexneri 3a crossreact with 10 other S. flexneri serotypes or subserotypes, S. flexneri 2a, S. flexneri 3a and S. flexneri 6 have been proposed as three components of a pentavalent vaccine (along with S. dysenteriae 1 and S. sonnei) that could confer broad protection. Cross-protection among S. flexneri subserotypes has been demonstrated in a guinea pig model123, but has yet to be tested in humans and remains a high priority123.
Figure 2.
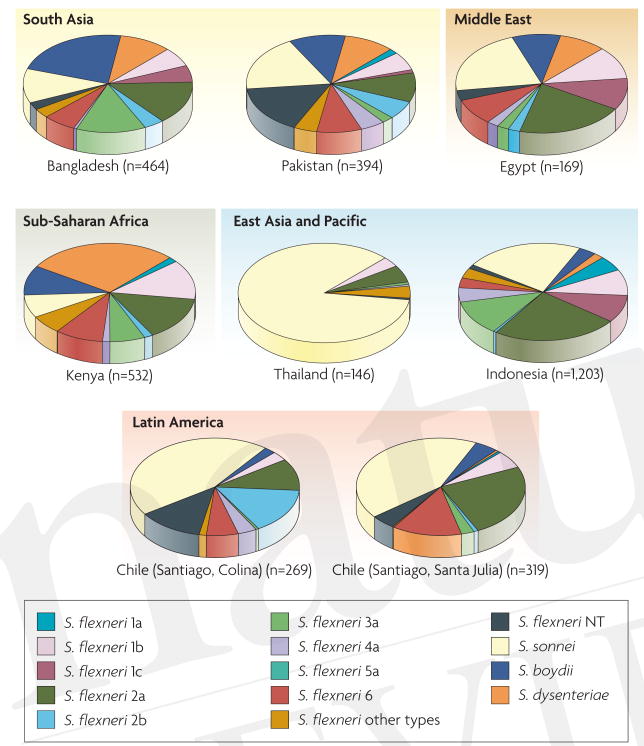
The distribution of Shigella species and Shigella flexneri serotypes from selected studies in Latin America, Asia and Africa. Several population-based surveillance studies lasting at least 2 years used extensive serotyping methods (including either commercial absorbed animal sera or monoclonal antibodies for Shigella flexneri typing)6–9,129–131. In all countries, S. flexneri 2a is the single most important S. flexneri subserotype. The most developed of the countries, Chile and Thailand, show a notable proportion of cases due to Shigella sonnei. In any individual country, 3–4 serotypes constitute ∼75% of all cases. However, the other important S. flexneri serotypes, in addition to S. flexneri 2a, differ from country to country. Serotype 1c described in some studies is a provisional serotype. If the CVD pentavalent vaccine strategy that incorporates S. flexneri 2a, S. flexneri 3a and S. flexneri 6 (along with Shigella dysenteriae 1 and S. sonnei) succeeds in conferring cross-protection against the other 11 classical S. flexneri serotypes in future field trials, it should be possible to achieve a broad-spectrum global vaccine against shigellosis.
Few bacterial pathogens have had their pathogenesis or interactions with mammalian tissues elucidated so precisely at the cellular and subcellular levels as Shigella spp.20,21 Nevertheless, progress in attaining safe and effective Shigella vaccines has faltered. Herein, we review recent and old clinical trials that have evaluated the safety, immunogenicity and efficacy of candidate Shigella vaccines. We relate the bassis for the most popular strategies (BOX 2), the relevance of the different immune responses measured, the factors that have favoured or impeded vaccine development and, most importantly, the lessons that must be learned for the future.
Box 2. Shigella vaccine strategies.
Strategies for which there is evidence from field trials that demonstrates efficacy:
Attenuated strains of Shigella used as live oral vaccines.
O polysaccharides of Shigella covalently linked to carrier proteins and used as parenteral conjugate vaccines.
Strategies that are being (or have been) pursued but for which evidence of protection in humans has not been documented:
Proteosomes (outer membrane vesicles of group B Neisseria meningitidis) to which Shigella sonnei or Shigella flexneri 2a is adsorbed124. This vaccine has been administered to humans intranasally.
Inactivated S. sonnei administered orally125.
Shigella invasion complex (Invaplex); a subcellular vaccine prepared from pathogenic S. flexneri, containing proteins including invasion plasmid antigen B (IpaB) and IpaC) and lipopolysaccharide, administered to humans intranasally126.
Nuclear protein–ribosomal parenteral vaccine prepared from a Shigella strain that has been genetically attenuated by an msbB mutation that partially detoxifies the lipid A portion of the Shigella endotoxin127,128.
Shigellosis models and vaccine development
The ability of Shigella to cause diarrhoeal illness is restricted to human and higher non-human primate (NHP) hosts. Experimental models of shigellosis in adult human volunteers and NHPs have been invaluable for studying Shigella pathogenesis, infection and immunity as well as for evaluating candidate Shigella vaccines. Epidemiologically, shigellosis is a low-inoculum infection that is readily transmitted by direct faecal–oral contact. This has been corroborated by volunteer studies in which the ingestion of as few as ten S. dysenteriae 1 organisms induced a clinical infection rate of 10%22 and 200 organisms of different serotypes caused infection rates that were >25%22–26. In a volunteer model of experimental shigellosis that was developed in the late 1960s, the Shigella inoculum was administered in skimmed milk without buffer22,23,26,27. However, this was accompanied by considerable variability in the infection rate, particularly at lower inocula levels. A helpful modification was to administer the inoculum with NaHCO3 buffer25, which achieved consistency in the rate of infection25,28 (TABLE 2). Volunteers develop classical bacillary dysentery, which commences with fever, malaise, abdominal cramps and (often watery) diarrhoea (for ∼18–24 hours). These symptoms are followed by scanty stools that contain blood and mucus and by tenesmus25. With early antibiotic therapy, volunteers rapidly respond clinically and microbiologically (their cultures are often negative within 24 hours)25.
Table 2. Response to wild-type Shigella flexneri 2a.
| Challenge | Adverse clinical outcomes | IgA anti-O-antigen ASC | |||
|---|---|---|---|---|---|
| Diarrhoea | Dysentery | Fever | % Responders | Geometric mean* | |
| Challenge no. 1 | 12/14 (86%) | 10/14 (71%) | 10/14 (71%) | 100% | 71 |
| Challenge no. 2 | 11/12 (92%) | 10/12 (83%) | 10/12 (83%) | 92% | 239 |
This Table shows the consistency of the clinical and immunological response in North American adult volunteers following challenge with 103 colony-forming units (CFU) of wild-type Shigella flexneri 2a strain 2457T administered with NaHCO3 buffer.
Per 106 PBMC. ASC, antibody-secreting cells; PBMC, peripheral blood mononuclear cells.
In contrast to the disease in humans, a large inoculum (∼ 1010 colony-forming units (CFU)) administered with NaHCO3 is required to reliably induce shigellosis in NHPs29–31. So, although the NHP model is helpful for studying pathogenesis, infection-derived immunity and perhaps the efficacy of vaccines, it is not useful for predicting the clinical acceptability of putatively attenuated live vaccine candidates for humans.
The gross pathology of shigellosis in the human colon and the terminal ileum reflects the mucosal invasiveness of this pathogen. Understandably, most work on the molecular pathogenesis of Shigella infections has focused on the genes and gene products that are involved in invading epithelial cells and the host response to invasion20,21. The early watery diarrhoeal phase of shigellosis was largely ignored until the NHP and volunteer model studies began to shed light on this stage of pathogenesis. Clinicians recognize that some patients with shigellosis manifest only watery diarrhoea, and even dysentery patients passing scanty bloody stools usually recount a day of watery diarrhoea without blood before the onset of their dysentery. When Rout et al.32 fed virulent S. flexneri 2a to NHPs, some NHPs developed only watery diarrhoea, whereas others developed only dysentery and some manifested both. In vivo perfusion studies revealed that colonic net transport of water occurred in all clinically ill NHPs, whereas only NHPs with watery diarrhoea exhibited net secretion of water, sodium and chloride ions in the jejunum, indicating the effect of an enterotoxin32. Histological examination demonstrated no jejunal abnormalities, corroborating the hypothesis that an enterotoxin (or toxins) was probably responsible for the watery diarrhoea. Kinsey et al.33 proved that the initiation of watery diarrhoea required that S. flexneri 2a be present in the jejunum. When they bypassed the jejunum and inoculated NHPs directly in the cecum, the animals developed dysentery but not watery diarrhoea, and no jejunal secretion was observed33. The early watery diarrhoea phase, which can clearly be discerned in the volunteer model of shigellosis, prompted Fasano et al. to look for enterotoxins that could elicit small intestinal secretion in the absence of invasion34–37. Their efforts led to the discovery and characterization of Shigella enterotoxin 1 (ShET1; encoded by the chromosomal gene set) and ShET2 (encoded by the plasmid-borne gene sen)34–37. ShET1, a classical enterotoxin formed by a single A subunit that combines with five B subunits, is encoded on a chromosomal pathogenicity island38 and is restricted to isolates of S. flexneri 2a37–41. By contrast, ShET2, which is encoded by a gene on an invasiveness plasmid, is expressed by all serotypes 36,39–41. The identification of these enterotoxins has had enormous repercussions for the development of well-tolerated live oral Shigella vaccine strains (TABLE 3).
Table 3. The role of the ShET1 and ShET2 enterotoxins.
| Strain | Adverse clinical outcomes | IgA anti-O-antigen ASC | ||||||
|---|---|---|---|---|---|---|---|---|
| Diarrhoea | Dysentery | Fever | % Responders | Geometric Mean* | ||||
| 108 | 109 | 108 | 109 | 108 | 109 | 109 | N/A | |
| ΔguaBA (CVD 1204) | 3/7 (43%) | 4/7 (57%) | 0/7 | 0/7 | 0/7 | 4/7 | 100% | 445 |
| ΔguaBA, Δset, Δsen, (CVD 1208) | 0/7 | 0/7 | 0/7 | 0/7 | 0/7 | 1/7 | 86% | 62 |
This Table shows the consistency of the clinical and immunological response in North American adult volunteers to ingestion of 108 or 109 colony-forming units (CFU) of two deletion mutants of wild-type S. flexneri 2a strain 2457T with NaHCO3 buffer.
Per 106 PBMC. ASC, antibody-secreting cells; PBMC, peripheral blood mononulcear cells; ShET, Shigella enterotoxin.
Immunity following wild-type infection
Convincing evidence that an initial clinical Shigella infection elicits serotype-homologous protection comes from three sources: NHP challenge studies31, volunteer model re-challenge studies24,25,42 and prospective epidemiological surveillance of a cohort of children in an endemic area6. Formal et al. reported that an initial S. flexneri 2a diarrhoeal infection in NHPs conferred 100% protection against re-challenge with S. flexneri 2a, but offered no protection against challenge with S. sonnei31. For 30 months, Ferreccio et al. prospectively followed a cohort of children <4 years of age in a poor community in Santiago, Chile, where shigellosis was endemic. They found that 3 serotypes, S. sonnei, S. flexneri 2a and S. flexneri 6, caused 79% of the cases of shigellosis6. As these serotypes do not share O-antigen determinants (BOX 1), this provided a unique opportunity to investigate the immunity that follows natural clinical exposure to each of these serotypes. By comparing the incidence of subsequent episodes of shigellosis caused by a homologous serotype against the overall incidence of subsequent episodes, it was calculated that an initial bout of shigellosis afforded 72% protection against a second clinical illness due to the homologous serotype (p=0.05)6. Protection against a second clinical illness due to a heterologous serotype was low (<30%; p = not significant). Collectively, these data suggest that antibodies to Shigella O-antigen have a key role in protection (FIG. 3).
Figure 3.
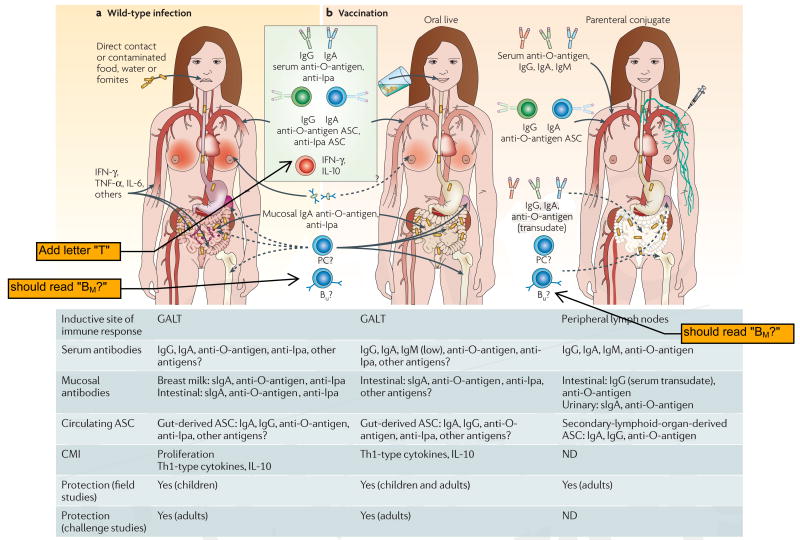
Immunity to Shigella. a | Immune responses to wild-type Shigella infection. The specific immune responses that mediate protection against shigellosis remain controversial. The strong serum IgG and IgA antibody responses to Shigella O-antigen induced by wild-type infection correlate with protection data from epidemiological6 and seroepidemiological studies111,132 as well as non-human primate challenges31. Gut-derived IgA anti-O-antigen antibody-secreting cell (ASC) responses are also thought to have a significant role in protection. By contrast, the role of antibody responses to Shigella protein antigens (such as invasion plasmid antigen (Ipa) proteins, VirG and others) and of cell-mediated immunity (CMI) in contributing to protection remain a matter of speculation. b | Immune responses to vaccines. The immune responses generated by types of Shigella vaccines (live oral and parenteral conjugate) that have been shown to confer protection are shown. Responses elicited by live oral vaccines include IgA and IgG anti-O-antigen and anti-Ipa ASC detected among peripheral blood mononuclear cells (PBMC), serum IgA and IgG anti-O-antigen and anti-Ipa, as well as IgA anti-O-antigen in stool, intestinal fluid and urine25,49,50,60,86. Interferon-γ(IFN-γ) responses to Shigella antigens have also been observed in volunteers immunized with Shigella flexneri 2a strain CVD 1207 (REF. 87), Shigella sonnei strain WRSS1 (REF 94) and SFL1070 (REF 85). The contribution of CMI and of antibodies to Shigella proteins (for example, Ipa and VirG) elicited by live oral vaccines in preventing clinical Shigella infection remains uncertain. In contrast to the broad immune responses elicited by live oral vaccines, parenteral conjugate vaccines elicit a narrower response, characterized predominantly by serum IgG antibodies to Shigella O-antigen. Wild-type Shigella or live oral vaccines are likely to generate long- and short-lived plasma cells (PC) and memory B cells (Bμ) that reside in the bone marrow, spleen and gut-associated lymphoid tissue (GALT). By contrast, PC and Bμ elicited by conjugate vaccines are likely to be confined to the bone marrow and spleen. The relative magnitudes of immune responses are depicted by gradients of colour at the various effector sites. IL, interleukin; ND, no data; TNF-α, tumour-necrosis factor-α.
The debate over whether protection is predominantly mediated by mucosal secretory IgA (sIgA) anti-O-antigen antibodies, by serum IgG antibodies or by both (perhaps with contributions from other immune effector mechanisms) is more contentious. Strong mucosal (faecal and urinary) sIgA anti-O-antigen antibody responses are observed following wild-type infection25,28,43–47 and experimental challenge25,28. Moreover, priming of the mucosal immune system following an initial wild-type Shigella infection has also been documented by enumerating gut-derived IgA anti-O-antigen antibody-secreting cells (ASCs)25,28,46,48 (FIG. 3). On the one hand, although IgA anti-O-antigen ASCs and serum anti-O-antigen IgG have generally been considered key elements for protection, some studies have failed to show an association between these responses and resistance to illness following challenge49,50. In volunteer re-challenge studies, the substantial clinical protection exhibited by veterans24,25,42 is accompanied by an ∼1 log reduction in the level of excretion of Shigella25. In one endemic situation, initial clinical infection by S. flexneri 2a was followed by repetitive asymptomatic infections by that serotype and ultimately by sterile intestinal immunity such that even asymptomatic carriage was no longer detected51. On the other hand, the importance of mucosal antibodies in preventing shigellosis was demonstrated by Tacket et al. who reported that passive oral administration of cow's milk immunoglobulin that contained high titres of anti-S. flexneri 2a antibodies protected volunteers from experimental challenge with wild-type S. flexneri 2a strain 2457T52. Notably, cow's milk immunoglobulin that contained low titres of anti-S. flexneri 2a antibodies did not provide protection52. Additional research is needed to define the roles of intestinal sIgA and serum IgG antibodies in mediating protection against Shigella.
Other immune effector mechanisms have also been described in shigellosis (FIG. 3). Increases in serum anti-Ipa (invasion plasmid antigen) antibodies have been demonstrated after natural disease and experimental challenge25,43,53, and in endemic areas the seroprevalence of anti-Ipa antibodies increases with age43,54. A few studies have also measured cell-mediated immunity (CMI) elicited by wild-type Shigella infection. Rectal biopsies from Bangladeshis during acute and convalescent S. flexneri and S. dysenteriae 1 infection revealed an increased frequency of cytokine-producing cells and mononuclear cell infiltration in the rectal mucosa55. Increased levels of interferon-γ (IFN-γ) in plasma and stool, upregulation of IFN-γ receptor expression in patients with shigellosis and increased IFN-γ production during convalescence suggest that this cytokine might have a role in both pathogenesis and protection55–59. Production of IFN-γ and interleukin-10 (IL-10), but not IL-4, IL-5, IL-12 or IL-15, upon stimulation with Shigella antigens (including Ipa) was demonstrated in peripheral blood mononuclear cells (PBMC) in volunteers challenged with modified virulent S. dysenteriae 1 strain SC595, indicating a predominant T helper1 (TH1)-type response60. Also, increased proportions of memory T cells (CD45RO+), the expression of T-cell activation molecules (for example, CD25, CD38, HLA-DR and CD54) and the expansion of defined T-cell receptor Vβ families have been reported in patients with shigellosis61,62. Despite these findings, the contribution to protection from Shigella that can be attributed to CMI or to antibodies directed at Shigella proteins (such as Ipa and VirG) rather than O polysaccharide antigens remains controversial (FIG. 3).
Protective prototype vaccines
Two prototype Shigella vaccines, one which uses attenuated strains as live oral vaccines and the other which uses parenteral conjugates of Shigella O polysaccharide covalently linked to a carrier protein, have conferred significant protection in controlled Phase III trials. As these vaccines are furthest along in development, and as the results of the multiple clinical trials that have been carried out collectively provide insights, they will be discussed in depth. Other publications address promising alternative strategies (BOX 2) to develop Shigella vaccines63.
In the 1960s, Mel of the Military Medical Institute, Belgrade, Yugoslavia (Serbia), serially passaged different Shigella serotypes on streptomycin-containing media until they became streptomycin-resistant and streptomycin-dependent (SmD; that is, they could not grow in the absence of exogenous streptomycin)64,65. These SmD strains lost their ability to cause purulent keratoconjunctivitis in guinea pigs, a reliable test for Shigella mucosal invasiveness, intercellular spread and propensity to cause inflammation66. At the time, the genetic changes responsible for the attenuation were unknown. In addition to testing the vaccines in adults and children in Yugoslavia67–70, SmD Shigella vaccines were tested in adults and children in high-risk institutions in the United States42,51,71–73. These vaccine strains proved to be well tolerated clinically when administered to adults, healthy children and even debilitated institutionalized children42,51,71–73, with the main adverse reaction being vomiting in a small percentage of recipients following administration of the first dose51,69–74. The vaccines were typically administered in an escalating (2×1010, 3×1010, 4×1010 and 4×1010 CFU) 4-dose regimen over 11 days. In controlled field trials, Mel et al. demonstrated the efficacy of the SmD vaccines69,70, showed that multiple strains could be mixed together in combination vaccines and reported that protection was serotype-specific68–70. Protection endured for 1 year following primary immunization of children; however, administration of a single booster extended protection for an additional year70. These pioneering studies, which involved ∼36,000 adult and paediatric subjects, provided proof of concept for future modern multivalent vaccines that aim to confer broad-spectrum protection. Unfortunately, the SmD vaccine strains had drawbacks that precluded them from becoming licensed for widespread use. In addition to the fact that the basis for the attenuation was unknown, occasional lots reverted to streptomycin independence (although the organisms remained unable to invade epithelium) and difficulties were encountered in large-scale manufacture and process control73,75.
S. flexneri 2a strain T32 is another pioneering vaccine strain developed by Istrati in Romania, where it was shown to be well tolerated and probably protective76,77. Subsequently, in randomized placebo-controlled field trials in China, Bingrui et al. reported that T32 conferred significant protection against S. flexneri 2a diarrhoea78. Surprisingly, the Chinese field trials also suggested that T32 conferred significant (albeit lower level) protection against shigellosis due to S. flexneri 1b and S. boydii 1–6. Investigators at the Walter Reed Army Institute of Research (WRAIR) revealed that T32 harboured a large deletion in the invasiveness plasmid. This deletion resulted in the loss of three loci, ipaBCDA, invA and virG (also known as icsA), which crippled the ability of this strain to invade epithelial cells79. Further investigation showed that the T32 plasmid deletion encompassed sen (which encodes ShET2); the chromosomal set gene (which encodes ShET1) remained intact39.
In S. sonnei, the rfb locus, which encodes O-antigen, is located on the form I invasiveness plasmid. Investigators at the Lanzhou Institute of Biological Products introduced an S. sonnei form I plasmid with deletions of ipa and virF into S. flexneri 2a T32 (REF. 80). Three doses (from 2×1010 to 5×1010 CFU) of the resultant hybrid strain expressing both S. flexneri 2a and S. sonnei O-antigens provided ∼65% protection against both S. flexneri 2a and S. sonnei diarrhoea80.
Formal et al. at WRAIR prepared a ‘mutant-hybrid’ S. flexneri 2a vaccine strain by selecting a colonial mutant that had lost its ability to invade intestinal epithelial cells81 and transferred into it the xylose–rhamnose region of E. coli (which diminishes the ability of Shigella to propagate in the lamina propria even if epithelial invasion occurs)82. In Phase I and Phase II clinical trials in adults and children in the United States71,72, the S. flexneri 2a mutant-hybrid vaccine was as well tolerated as Mel's SmD vaccines and conferred partial (albeit nonsignificant) protection against experimental challenge in adult volunteers42; by comparison, SmD S. flexneri 2a conferred significant protection42.
Building on the pioneering work of Mel and Istrati, researchers worldwide have used recombinant DNA technology and knowledge of the molecular pathogenesis of shigellosis to create modern live oral Shigella vaccine candidates that can overcome the drawbacks of the early strains. Progress has been steady but painstakingly slow, as unexpected obstacles have been encountered.
The importance of the parent strain
One lesson that has been learned in developing attenuated Shigella vaccines is the importance of starting with a wild-type parent strain of known virulence, ideally as assessed in volunteer studies. The paradigm is the array of derivatives of wild-type S. flexneri 2a strain 2457T, a parent strain known to be virulent in North American volunteers and to cause diarrhoea, fever and dysentery with an infection rate that increases with escalating doses25,49,50 (TABLE 2). Moreover, an initial clinical episode of 2457T-induced shigellosis stimulates immunity that confers ∼64–75% protection against illness upon subsequent re-challenge with this strain25,42. As the baseline virulence of the parent strain is well documented, the degree of attenuation achieved by putative attenuating mutations can be quantified (TABLES 2,3).
It is difficult, however, to draw conclusions about the ability of specific mutations to cause attenuation when they are introduced into wild-type parent strains that were either not tested clinically beforehand or, if tested, were found to be only minimally pathogenic in humans. Two illustrative examples are wild-type S. flexneri Y strain SF1 and a Shiga-toxin-deleted derivative of wild-type S. dysenteriae 1 strain SC595 (which retains epithelial cell invasiveness and causes keratoconjunctivitis in guinea pigs). In the case of the S. flexneri Y strain SF1, an aroD mutation was introduced, resulting in attenuated putative vaccine candidate SFL124 (REF. 83). Subsequently, however, when the wild-type S. flexneri Y parent strain SF1 was fed to North American volunteers, it was only minimally pathogenic84. So, it was not possible to attribute the clinical behaviour of the vaccine strain to the introduced mutation. By contrast, when an aroD deletion was introduced into the well-characterized wild-type S. flexneri 2a strain 2457T, resulting in vaccine strain SFL1070, it was clearly demonstrated that this mutation afforded measurable attenuation85. Similarly, S. dysenteriae 1 strain SC595 caused only low rates of mild diarrhoeal illness when administered to North American volunteers (except in one subject who developed dysentery)60. SC595 did, however, elicit robust antibody and CMI responses following ingestion of as few as 300 CFU60. Not surprisingly, SC599, a derivative of SC595 that contains deletions in icsA (also known as virG; deletion diminishes the intracellular and intercellular spread of Shigella), ent and fep (which encodes proteins involved in iron chelation) was well tolerated in Phase I clinical trials63. However, SC599 was only minimally excreted and induced only modest anti-O-antigen ASC responses and few anti-O-antigen seroconversions63.
Selection of attenuating mutations
The phenotypic consequences of introducing different mutations into wild-type S. flexneri 2a strains have been evaluated in Phase I clinical trials. These mutations include aroD and aroA (which encode enzymes in the aromatic amino-acid biosynthesis pathway; mutation makes mutants auxotrophic for substrates not found in sufficient concentration in mammalian tissues)85,86, virG28,87, iuc (which encodes aerobactin)28, guaBA (which encodes enzymes involved in guanine-nucleotide biosynthesis)87,88, set (which encodes ShET1) and sen (which encodes ShET2)87,88.
The experience with strain SC602, a derivative of wild-type strain Pasteur Institute S. flexneri 2a 494 with mutations in virG and iuc, is instructive28,89. When fed to North American volunteers, dosages ≥106 CFU were unacceptably reactogenic, with most subjects developing diarrhoea, fever or severe constitutional symptoms, although the vaccine strain was heavily shed and highly immunogenic28. By contrast, at a dosage level of 104 CFU, adverse clinical reactions were uncommon and mild, yet the induced immune response remained moderately robust28. Eight weeks after vaccinees received a single 104 CFU dose of SC602, 7 vaccinees (and seven unvaccinated control volunteers) participated in an experimental challenge study and ingested 103 CFU of virulent S. flexneri 2a strain 2457T28. Diarrhoea was observed in 86% of the controls and in 43% of the vaccinees (p=0.27)28. However, the 3 vaccinees that developed diarrhoea had significantly milder illness than the controls. Four of the 7 controls (57%) developed dysentery, 6 (86%) had fevers (mean temperature 39.28 °C) and 6 (86%) manifested severe shigellosis. By contrast, none of the vaccinees had dysentery, fever or severe illness (p=0.0027)28.
This hallmark study showed that in the experimental challenge model even a single dose of an engineered vaccine strain could confer significant protection against severe shigellosis. However, it also demonstrated the difficulty of finding a proper balance between clinical acceptability and immunogenicity in adult volunteers in developed countries. Indeed, additional studies with SC602 in 34 community volunteers showed that 104 CFU caused mild diarrhoea in 5 volunteers (15%) and fever in 4 volunteers (12%)89.
Invaluable lessons have also been learnt from clinical trials with the series of attenuated derivatives of wild-type S. flexneri 2a strain 2457T that was developed by the University of Maryland Center for Vaccine Development (CVD), as the results have directed iterations in vaccine design. CVD 1203 is a derivative of 2457T that harbours deletions in aroA and virG90. In a Phase I clinical trial in 10 North American adults, CVD 1203 was well tolerated at a dose of 106 CFU (a dosage level at which SC602 was extremely reactogenic)86. However, when administered at doses of 108 CFU or 109 CFU86, CVD 1203 induced unacceptable reactogenicity accompanied by strong immune responses86. Significant increases in the concentrations of tumour-necrosis factor-α (TNF-α) were observed in serum and stool, suggesting that this cytokine might be related to the reactogenicity observed at high doses86. Although the potent immune responses to CVD 1203 were encouraging, the reactogenicity dictated that alternative mutations should be evaluated to achieve a satisfactory degree of attenuation.
Strain CVD 1207 harbours deletion mutations in guaBA91, virG90, set34 and sen36. When volunteers ingested escalating single doses of up to 1010 CFU of CVD 1207 (REF. 87), this live vaccine strain was remarkably well tolerated. No subjects manifested adverse reactions through the 108 CFU dosage level87, and even at 109 and 1010 CFU only a few volunteers experienced mild diarrhoea87. The conclusion from the Phase I clinical trial of CVD 1207 was that the mutations definitively attenuated the 2457T parent strain, resulting in a vaccine that was well tolerated by North American volunteers at high dosage levels yet remained immunogenic. However, it was proposed that strain CVD 1207 could have been hyperattenuated87.
CVD 1204 and CVD 1208, isogenic derivatives of wild-type strain 2457T, both carry deletions in guaBA88,91, and CVD 1208 also has deletions in sen and set. These strains differ from isogenic CVD 1207 by having an intact virG gene. This design permitted researchers to assess the impact of inactivating ShET1 and ShET2 (TABLE 3). A randomized, double-blind clinical trial was performed in adult North Americans who ingested a single dose containing 107, 108 or 109 CFU of either strain88. Objective adverse reactions were observed in 35% of CVD 1204 recipients but only in 4% of CVD 1208 recipients (p=0.02)88. Immune responses to S. flexneri 2a O-antigen (ASCs, serum IgG or faecal IgA) were observed in 86%, 43% and 100% of CVD 1208 recipients, respectively. The enterotoxin-negative strain CVD 1208 was considered a highly attractive vaccine candidate that reflects the desired balance of clinical acceptability and robust immunogenicity (TABLE 3). As this strain was constructed using culture media that contained animal products, it was reformulated using soy-based media for regulatory purposes, resulting in strain CVD 1208S. In a Phase I clinical trial, CVD 1208S demonstrated the same clinical acceptability, shedding pattern and immunogenicity as CVD 1208 (K.L.K., unpublished data). Thus, CVD 1208S, like CVD 1208, exhibits the desirable balance of low reactogenicity and robust immunogenicity.
Investigators at WRAIR constructed strain WRSS1 by deleting virG in the wild-type S. sonnei Moseley strain92. Although the virulence of this S. sonnei strain had not been demonstrated in volunteers, it was selected because its form I invasiveness plasmid (required for expression of O-antigen by this serotype) was remarkably stable, in contrast with the form I plasmid of most wild-type S. sonnei strains24,93. In a Phase I trial in North American volunteers given a single dose of up to 106 CFU, low-grade fever or mild diarrhoea was recorded in 6 of 27 (22%) WRSS1 vaccinees94. Strong IgA anti-O-antigen ASC responses were observed in recipients of all dosage levels94. Moderate IFN-γ responses were also observed in some volunteers94.
Safety and immunogenicity trials of WRSS1 were next performed in Israel in community adults who ingested 103, 104 or 105 CFU95. At the two lower dosage levels, the vaccine was well tolerated with the exception of 1 of 30 subjects (3%) who developed moderate diarrhoea, and 5 (17%) who experienced mild diarrhoea. By contrast, at the 105 CFU dosage level, 2 of 15 subjects (13%) developed fever and 4 (27%) experienced moderate diarrhoea95. The 104 CFU dosage level provided the better balance of immunogenicity and clinical acceptability, as all subjects manifested IgA anti-O-antigen ASC responses and 73% of the vaccinees showed more than 50 IgA anti-O-antigen ASCs per 106 PBMC95.
So, certain attenuating mutations introduced into wild-type strains in recent years have generated promising vaccine candidates. Some modifications, for example deletion of the genes that encode ShET1 and ShET2, might work well in combination with an array of other attenuating mutations. If commercial and intellectual property considerations do not create barriers, it would be of interest to ‘mix and match’ novel combinations of attenuating mutations that have not previously been used in the same strains. For example, in their latest generation of live vaccine candidates, WRAIR investigators have deleted the ShET-encoding genes in strains that carry a virG deletion as the primary attenuating mutation (M. Venketasan, personal communication). Similarly, it would be instructive to directly compare the clinical acceptability and immunogenicity of two or more candidate live vaccine strains of the same serotype in randomized, double-blind clinical trials. Last, it has generally been proposed that once attenuating mutations have been documented in one serotype, they can be introduced into other epidemiologically relevant serotypes. However, if the commercial considerations can be managed, another track towards licensure of a broadly protective vaccine would be to combine promising strains of several different serotypes that have been attenuated by distinct mutations.
Hybrid live vector Shigella vaccines
As an alternative strategy to construct live Shigella vaccines, several research groups have attempted to express Shigella O- and other (Ipa) antigens in well-tolerated live vectors such as normal flora, rough E. coli or highly attenuated Salmonella enterica serovar Typhi. The genes that allow synthesis of group- and type-specific O-antigens of S. flexneri 2a were introduced into an E. coli O8 strain, resulting in the vaccine strain PGAI 42-1-15. This hybrid E. coli expressing S. flexneri 2a O-antigen was well tolerated when given to adult US volunteers and was excreted for several days27. In two separate large experimental challenge studies, volunteers given two (first study) or three (second study) doses of vaccine one month apart were challenged 4–8 weeks later with 104 CFU (first study) or 102 CFU (second study) of virulent S. flexneri 2a strain 2457T27. The rates of shigellosis were similar in vaccinees and controls27. It was unclear why this live vaccine failed to protect. However, one hypothesis is that to be protective, an E. coli live vector strain expressing Shigella O-antigens must also exhibit the capacity to invade epithelial cells27. Accordingly, investigators at WRAIR constructed two E. coli live vector strains expressing S. flexneri 2a O-antigen that manifested epithelial cell invasiveness.
The invasiveness plasmid of S. flexneri 5 and the genes that allow expression of S. flexneri 2a type and group-specific O-antigens were introduced into E. coli K12, producing a strain, EcSf2a-1, that could invade epithelial cells49. At a dose of 109 CFU, this strain was unacceptably reactogenic as 4 of 13 (31%) vaccinees developed fever, diarrhoea or dysentery. However, at lower, better-tolerated dosage levels, the vaccine failed to protect vaccinees against experimental challenge with S. flexneri 2a49. In an attempt to diminish reactogenicity, an aroD deletion was introduced, resulting in the further derivative strain EcSf2a-2 (REF. 96). At high dosage levels, this strain, which retained the ability to invade epithelial cells, also caused adverse reactions but was strongly immunogenic. However, despite robust immunogenicity, the vaccine conferred only 36% protection against illness (fever, diarrhoea or dysentery) upon experimental challenge (p=0.17)50. Overall, E. coli, with or without epithelial invasiveness, has been unsuccessful as a live vector vaccine expressing Shigella O-antigen.
In the 1980s, Formal and co-workers at WRAIR developed a vaccine candidate that consisted of licensed oral Salmonella Typhi live vaccine strain Ty21a engineered to express the O polysaccharide of S. sonnei97. This live vector strain, 5076-1C, was well tolerated but exhibited lot-to-lot variability in its immunogenicity and ability to protect in challenge studies in which vaccinees and controls ingested wild-type S. sonnei strain 53G24,48,98; however, two pilot lots did confer significant protection93. Multiple hypotheses were pursued to explain the lot-to-lot variability in efficacy99–102, but none were predictive and further development of 5076-1C was abandoned24.
To overcome the inconsistency of 5076-1C, Kopecko and co-workers attempted to more reliably express S. sonnei O-antigen in Ty21a, as well as O-antigens from other serotypes103. As Salmonella has a lipopolysaccharide (LPS) core that is chemically distinct from that of Shigella103, S. sonnei O polysaccharide in Salmonella Typhi is expressed only as a non-core-linked surface-associated antigen103. Clinical trials are required to assess if these new Ty21a-based constructs will offer more consistent protection.
Intestinal barrier in developing country populations
Oral vaccines against polio, rotavirus and cholera have exhibited lower immunogenicity in underprivileged subjects in developing countries compared with subjects in developed countries104–107. The immunogenicity of some of these vaccines was successfully enhanced by increasing the number of vaccine organisms per dose or by administering additional doses of vaccine104,106. Although S. flexneri 2a vaccine strain SC602 had been reactogenic in North American adults, as it conferred protection against severe disease in an experimental challenge, Phase I and II trials were undertaken in adults and children in a developing country. None of 68 Bangladeshi adults who ingested up to 106 CFU of SC602 experienced adverse reactions, but neither did any vaccinee shed SC602, and the immune responses induced were meager63. Next, Bangladeshi children of 8–10 years of age and of 12–36 months of age ingested up to 106 CFU63. Although no child manifested an adverse reaction, neither did any child excrete the vaccine strain or mount a significant immune response. So, the developing country subjects manifested markedly different clinical, bacteriological and immunological responses to SC602 compared with North American subjects. In adults in the United States, a low dose (104 CFU) of SC602 was reactogenic, heavily excreted and moderately immunogenic, whereas in the children and adults in Bangladesh it was virtually inert, even in a 100-fold higher dose63. One possible hypothesis to explain these divergent results is that Bangladeshis have decreased levels of accessible iron in tissues compared with subjects in developed countries, so that SC602 is more crippled in individuals in the developing countries.
Does this mean that most live oral Shigella vaccines will be poorly immunogenic in developing country populations? The response of Vietnamese adults83 and children108 to the ingestion of S. flexneri Y strain SFL124 (an aroD-deletion mutant) and the efficacy of the SmD and T32 live oral Shigella vaccines in subjects in Yugoslavia (late 1960s and early 1970s)69,70 and China (1980s)78, respectively, suggest not. A single 2×109 CFU dose of SFL124 was administered to 15 Vietnamese adult volunteers, whereas another 15 subjects ingested three doses (at an interval of two days between doses)83. No subjects manifested fever or diarrhoea, SFL124 was excreted by all volunteers and was immunogenic. Although few subjects excreted the strain, SFL124 was also immunogenic in Vietnamese children of 9–14 years of age and stimulated local mucosal immune responses and ASC responses to S. flexneri Y O- and Ipa antigens in a dose-dependent manner108. The high baseline titres of serum antibodies to S. flexneri Y O- and Ipa antigens in the Vietnamese children indicate that the vaccine was seen as a booster rather than as a primary immunogen. It is not clear whether the difference in the immune responses to SC602 and SFL124 live oral Shigella vaccines in developing country populations is due to differences in the populations that participate in the clinical trials, to the inherent immunogenicity of the two strains or to disparities in the immunological assays.
Shigella conjugate vaccines
Investigators at the National Institute of Child Health and Human Development (NICHD) in the United States have proposed that in subjects immune to Shigella small amounts of serum IgG transude onto the gut surface, where they can neutralize or otherwise inactivate inocula of wild-type Shigella organisms shortly after ingestion109,110 (FIG. 3). On the basis of this concept, NICHD investigators have developed parenteral conjugate vaccines that consist of O polysaccharides derived from the LPS of relevant Shigella serotypes covalently linked to a carrier protein (Pseudomonas aeruginosa exotoxin A (PsA) or CRM9-mutant diphtheria toxin). Data supporting the concept that serum IgG anti-O-antigen antibodies correlate with (and might mediate) protection come from the seroepide-miological studies of Cohen and colleagues111,112. Baseline serum specimens were obtained from Israeli military conscripts upon deployment to training bases where they were exposed to a high risk of shigellosis111,112. The rates of S. sonnei and S. flexneri 2a disease were significantly lower in individuals who had elevated non-IgM anti-O-antigen titres at baseline. Additional supporting data come from the studies of Black et al., who showed that North American volunteers with elevated serum IgA and IgG anti-S. sonnei-O-antigen antibodies following oral immunization with attenuated Salmonella Typhi expressing S. sonnei O-antigen had lower infection rates when challenged with virulent S. sonnei in experimental challenge studies93.
A parenteral prototype NICHD vaccine that consists of S. sonnei O polysaccharide conjugated to PsA was shown to be well tolerated and highly immunogenic in stimulating serum IgG O-antigen antibodies in young Israeli soldiers113,114. In a randomized, controlled, double-blind Phase III efficacy trial involving several hundred Israeli soldiers, a single dose of the S. sonnei conjugate vaccine conferred 74% protection against S. sonnei diarrhoea during outbreaks on army bases114. The efficacy of the vaccine was related to the level of conjugate-induced IgG anti-O-antigen antibody.
Phase II trials assessed the clinical acceptability and immunogenicity of S. sonnei (conjugated to rPsA or CRM9) and S. flexneri 2a (conjugated to rPsA) vaccines in Israeli children that were 4–7 and 1–4 years of age115,116. In these paediatric studies, a two-dose immunization schedule was used with doses spaced 6 weeks apart115,116. The first injection of each vaccine stimulated a significant (9–13-fold) rise in homologous serum IgG anti-O-antigen antibodies116. In 1–4-year-olds, the second injection stimulated a booster response in both recipients of S. sonnei (2.9-fold) and S. flexneri 2a (1.6-fold) conjugates; the titres remained elevated 2 years after vaccination116.
A Phase III randomized trial of S. sonnei conjugate and S. flexneri 2a conjugate vaccines, with each serving as a control for the other, was recently completed in paediatric subjects in Israel. The results of this trial will determine whether the S. sonnei conjugate vaccine can protect young children. The next generation of Shigella conjugates, based on synthetic oligosaccharides linked to proteins, offers promise for enhanced immunogenicity and diminished production costs117.
Strategies to achieve broad-spectrum protection
Arguably the greatest impediment to achieving a useful Shigella vaccine is devising a strategy that can confer broad protection against a large number of epidemiologically relevant serotypes. There is widespread agreement that a global Shigella vaccine must protect against 16 serotypes and subserotypes, including S. dysenteriae 1, S. sonnei and all 14 classical S. flexneri types and subserotypes (TABLE 1; BOX 1; FIG. 2). Yet a vaccine containing so many strains or conjugates would be impractical and expensive, even if it could be formulated. To resolve this dilemma with a ‘pentavalent strategy’, researchers at the CVD included S. flexneri 2a, S. flexneri 3a and S. flexneri 6 strains along with the attenuated S. dysenteriae 1 and S. sonnei strains in a multivalent vaccine, because these 3 S. flexneri serotypes have O-antigen group determinants that are shared by the remaining 11 S. flexneri serotypes and subserotypes118 (BOX 1). CVD investigators have demonstrated in a guinea pig model that a multivalent vaccine containing serotypes S. flexneri 2a and S. flexneri 3a provides an impressive level of cross-protection against other S. flexneri serotypes that share the common O-antigen epitopes118.
As Ipa and VirG proteins are plasmid-encoded virulence attributes that are expressed by all virulent Shigella, irrespective of serotype, it is attractive to propose basing broad protection on stimulating serological and CMI responses to these (and perhaps to other common protein) antigens (FIG. 3). However, such responses will have to be even stronger than those elicited by wild-type infection (FIG. 3). This is because NHP challenges and epidemiological data suggest that protection due to wild-type infection does not confer serogroup-heterologous cross-protection, despite eliciting serological responses to Ipa proteins53.
The way forward
Identification of the correlates of protection is arguably the most crucial catalyst needed to accelerate the development of effective Shigella vaccines. The strongest immunizing regimens that confer protection (wild-type infection and live oral vaccines) appear to mediate protection via a confluence of immune responses. These include systemic and mucosal antibody responses to Shigella O- (and other) antigens, cytokines (particularly IFN-γ, secreted early as part of the innate immune response and later by specific T cells) and other CMI responses. Correlation of the panoply of effector immune responses elicited in subjects immunized with Shigella vaccines, exposed to wild-type Shigella and protected due to exposure to wild-type Shigella will help us identify the key immune responses that afford strong, long-lasting protection from disease. Another catalyst required to accelerate Shigella vaccine development is increased financial support for clinical trials (including challenge studies) to evaluate vaccine candidates and elucidate correlates of protection.
Although attractive candidate vaccines have entered clinical development, the road ahead presents barriers to be overcome. Whether the pentavalent strategy of the CVD can provide broad cross-protection in humans remains an important question. Also, we must document whether Shigella vaccines — live oral or parenteral conjugate — can protect vulnerable immunologically naive paediatric populations in developing countries as well as travellers from developed countries. Once vaccines have been developed that overcome the biological and epidemiological hurdles and result in licensed products, realization of the potential of vaccines as public health tools will require that yet other hurdles be surmounted, including generating political will and devising financial solutions to assure the introduction and programmatic use of vaccines in developing countries. The advent of the Global Alliance for Vaccines and Immunisation (GAVI) and its Fund, Advanced Market Commitments, the International Finance Facility for Immunisation and other innovative tools for financing the purchase of vaccines for the poorest developing countries offer tangible solutions that were unimaginable a decade ago. These innovations offer optimism that the fruits of microbiological and immunological research, such as the development of well tolerated, effective Shigella vaccines, can reach the neediest target populations.
Acknowledgments
This paper includes work funded, in part, by the National Institute of Allergy and Infectious disease (NIAID), the National Institutes of Health (NIH), Department of Health and Human Services (DHHS) federal research grants and contracts N01 AI25461, R01 AI029471 and U54 AI57168 (to M.L.), R01 AI57927 and NO1 AI30028 (to M.S.) and R01 AI059223 (to E.B.) and a grant from the Bill and Melinda Gates Foundation (to M.L.).
Biographies
Biographies
Myron M. Levine, vaccinologist, epidemiologist and microbiologist, is the Grollman Distinguished Professor of Medicine and Director of the Center for Vaccine Development (CVD) at the University of Maryland School of Medicine, USA. He received his M.D. from the Medical College of Virginia, USA, and his D.T.P.H. from the London School of Hygiene and Tropical Medicine, UK. Over 4 decades, he has studied the epidemiology and pathogenesis of Shigella infections and has developed and tested Shigella vaccines. A member of the Institute of Medicine, past president of the American Epidemiological Society and of the American Society of Tropical Medicine and Hygiene, he received the Albert B. Sabin Gold Medal for lifetime achievement in vaccine development.
Karen Kotloff received her M.D. from Temple University School of Medicine, Philadelphia, USA. Her specialty is Paediatric Infectious Diseases. Kotloff is currently Professor of Pediatrics and Chief of the Community Studies Section at the CVD. She is an expert in shigellosis and clinical evaluation of Shigella vaccines, including in-patient challenge studies to determine the protective efficacy of Shigella vaccine candidates. She is actively involved in epidemiological and clinical studies in Mali, West Africa, and is currently director of clinical and epidemiology activities in a multicentre epidemiological study to determine the aetiology of childhood diarrhoeal diseases in eight developing countries in Sub-Saharan Africa and South Asia.
Eileen Barry holds a Ph.D. from the Medical College of Virginia, Virginia Commonwealth University, USA. She is presently Associate Professor of Medicine and Chief of the Shigella Vaccine Section at the CVD. Her research focuses on the development of new live attenuated Shigella strains and multivalent Salmonella and Shigella live vector vaccine candidates. She is currently working in the development of a combined Shigella–Escherichia coli vaccine for travellers and children in developing countries in which both diseases are endemic.
Marcela Pasetti received a Ph.D. from the University of Buenos Aires, Argentina. She is currently Assistant Professor of Pediatrics and Chief of the Applied Immunology Section at the CVD. Her research interest is in paediatric vaccines and immune responses in newborns and young infants. She has conducted pre-clinical studies evaluating Salmonella and Shigella strains expressing foreign antigens or carrying DNA vaccines in animal models including mice, cotton rats and non-human primates. Her laboratory measures B-cell responses for CVD vaccine trials and has produced the immunological read-outs in recent clinical studies evaluating new live Shigella vaccine candidates in humans.
Marcelo B. Sztein holds faculty appointments as Professor in the Departments of Pediatrics, Medicine and Microbiology and Immunology at the University of Maryland, USA. Sztein is the Leader of the Immunology Group and Chief of the Cellular Immunology Section and Flow Cytometry Core Laboratory at the CVD. He received his M.D. degree from the University of Buenos Aires. For over 2 decades Sztein's research has focused on studies in humans and animal models to uncover the immune mechanisms of protection against infectious agents with the long-term goal of accelerating the development of effective vaccines. His main areas of interest include shigellosis, typhoid fever, malaria, tularemia, hepatitis B and influenza.
TOC
Shigellosis still causes a huge burden of disease worldwide, particularly in infants and young children in developing countries. Yet despite more than 4 decades of intensive research, efforts to develop a shigellosis vaccine have been unsuccessful. Here, Levine and colleagues review the story so far.
Footnotes
Competing interests statement: The authors declare no competing financial interests.
Links
Entrez Genome Project:
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD=search&DB=genomeprj
Salmonella enterica
Shigella dysenteriae
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=genomeprj&cmd=Retrieve&dopt=Overview&list_uids=13143
Shigella flexneri
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=genomeprj&cmd=Retrieve&dopt=Overview&list_uids=12334
Shigella sonnei
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=genomeprj&cmd=Retrieve&dopt=Overview&list_uids=13149
Entrez Gene: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=geneVirG
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=gene&cmd=Retrieve&dopt=full_report&list_uids=876473
UniProtKB: http://ca.expasy.org/sprot
IFN-γ
http://ca.expasy.org/uniprot/P01579
IL-10
http://ca.expasy.org/uniprot/P22301
TNF-α
http://ca.expasy.org/uniprot/P01375
Eileen Barry's homepage: http://medschool.umaryland.edu/CVD/Fac_Res_Interest.asp?id=38
Karen Kotloff's homepage: http://www.umm.edu/doctors/karen_l_kotloff.html
Marcela Pasetti's homepage: http://medschool.umaryland.edu/facultyresearchprofile/viewprofile. aspx?id=4848
Marcelo Sztein's homepage: http://medschool.umaryland.edu/CVD/Fac_Res_Interest.asp?id=52
Myron Levine's homepage: http://www.umm.edu/doctors/myron_m_levine.html
Center for Vaccine Development: http://medschool.umaryland.edu/CVD
Global Alliance for Vaccines and Immunisation: http://www.gavialliance.org
International Finance Facility for Immunisation: http://www.iff-immunisation.org
References
- 1.Shiga K. The trend of prevention, therapy and epidemiology of dysentery since the discovery of its causative organism. N Eng J Med. 1936;215:1205–1211. [Google Scholar]; Musings on a lifetime of study ofShigella infections by the discoverer of this pathogen.
- 2.Shiga K. Über den Dysenteribacillus (Bacillus dysenteriae). Zentrablatt für Bakteriologie, Parasitenkunde u Infektionskrankheiten Erste Abteilung: Medicinisch-hygienische. Bakteriologie und tierische Parasitenkunde. 1898;24:817–818. [Google Scholar]
- 3.Yabuuchi E. Bacillus dysentericus(sic) 1897 was the first taxonomic rather than Bacillus dysenteriae 1898. Int J Syst Evol Microbiol. 2002;52:1041. doi: 10.1099/00207713-52-3-1041. [DOI] [PubMed] [Google Scholar]
- 4.Strockbine NA, Maurelli AT. In: Bergey's Manual of Systematic Bacteriology. Brenner DJ, Krieg NR, Staley TE, editors. Springer; New York: 2005. pp. 811–823. [Google Scholar]
- 5.Kotloff KL, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77:651–666. [PMC free article] [PubMed] [Google Scholar]; A review of the epidemiological importance ofShigella infections.
- 6.Ferreccio C, et al. Epidemiologic patterns of acute diarrhea and endemic Shigella infections in a poor periurban setting in Santiago, Chile. Am J Epidemiol. 1991;134:614–627. doi: 10.1093/oxfordjournals.aje.a116134. [DOI] [PubMed] [Google Scholar]; The epidemiological study of paediatric shigellosis shows that under natural conditions of exposure, a prior clinicalShigella infection confers ∼72% protection against subsequent disease upon exposure to a homologous serotype.
- 7.von Seidlein L, et al. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. Plos Med. 2006;3:e353. doi: 10.1371/journal.pmed.0030353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutta P, et al. Assessing the cause of in-patients pediatric diarrheal deaths: an analysis of hospital records. Indian Pediatr. 1995;32:313–321. [PubMed] [Google Scholar]
- 9.Brooks JT, et al. Epidemiology of sporadic bloody diarrhea in rural Western Kenya. Am J Trop Med Hyg. 2003;68:671–677. [PubMed] [Google Scholar]
- 10.Fulla N, Prado V, Duran C, Lagos R, Levine MM. Surveillance for antimicrobial resistance profiles among Shigella species isolated from a semirural community in the northern administrative area of Santiago, Chile. Am J Trop Med Hyg. 2005;72:851–854. [PubMed] [Google Scholar]
- 11.Mata L, Gangarosa E, Caceres A, Perera D, Mejicanos M. Epidemic Shiga bacilllus dysentery in Central America. I. Etiologic investigations in Guatemala, 1969. J Infect Dis. 1970;122:170–180. doi: 10.1093/infdis/122.3.170. [DOI] [PubMed] [Google Scholar]; Epidemiological and microbiological studies at the onset of the Central American pandemic of Shiga dysentery in the late 1960s.
- 12.Rahaman MM, Khan MM, Aziz KMS, Islam MS, Kibriya AK. An outbreak of dysentery caused by Shigella dysenteriae type 1 on a Coral Island in the Bay of Bengal. J Infect Dis. 1975;132:15–19. doi: 10.1093/infdis/132.1.15. [DOI] [PubMed] [Google Scholar]
- 13.Ebright JR, et al. Epidemic Shiga bacillus dysentery in Central Africa. Am J Trop Med Hyg. 1984;33:1192–1197. doi: 10.4269/ajtmh.1984.33.1192. [DOI] [PubMed] [Google Scholar]
- 14.Aragon M, Barreto A, Chambule J, Noya A, Tallarico M. Shigellosis in Mozambique: the 1993 outbreak rehabilitation — a follow-up study. Trop Doct. 1995;25:159–162. doi: 10.1177/004947559502500405. [DOI] [PubMed] [Google Scholar]
- 15.Birmingham ME, Lee LA, Ntakibirora M, Bizimana F, Deming MS. A household survey of dysentery in Burundi: implications for the current pandemic in sub-Saharan Africa. Bull World Health Organ. 1997;75:45–53. [PMC free article] [PubMed] [Google Scholar]
- 16.Shane AL, Tucker NA, Crump JA, Mintz ED, Painter JA. Sharing Shigella: risk factors for a multicommunity outbreak of shigellosis. Arch Pediatr Adolesc Med. 2003;157:601–603. doi: 10.1001/archpedi.157.6.601-b. [DOI] [PubMed] [Google Scholar]
- 17.Mohle-Boetani JC, et al. Communitywide shigellosis: control of an outbreak and risk factors in child day-care centers. Am J Public Health. 1995;85:812–816. doi: 10.2105/ajph.85.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyams KC, et al. Diarrheal disease during Operation Desert Shield. N Engl J Med. 1991;325:1423–1428. doi: 10.1056/NEJM199111143252006. [DOI] [PubMed] [Google Scholar]
- 19.Levine MM. Immunization against bacterial diseases of the intestine. J Pediatr Gastroenterol Nutr. 2000;31:336–355. doi: 10.1097/00005176-200010000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Cossart P, Sansonetti PJ. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science. 2004;304:242–248. doi: 10.1126/science.1090124. [DOI] [PubMed] [Google Scholar]
- 21.Phalipon A, Sansonetti PJ. Shigella's ways of manipulating the host intestinal innate and adaptive immune system: a tool box for survival? Immunol Cell Biol. 2007;85:119–129. doi: 10.1038/sj.icb7100025. [DOI] [PubMed] [Google Scholar]
- 22.Levine MM, et al. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis. 1973;127:261–270. doi: 10.1093/infdis/127.3.261. [DOI] [PubMed] [Google Scholar]
- 23.DuPont HL, Hornick RB, Dawkins AT, Snyder MJ, Formal SB. The response of man to virulent Shigella flexneri 2a. J Infect Dis. 1969;119:296–299. doi: 10.1093/infdis/119.3.296. [DOI] [PubMed] [Google Scholar]
- 24.Herrington DA, et al. Studies in volunteers to evaluate candidate Shigella vaccines: further experience with a bivalent Salmonella typhi–Shigella sonnei vaccine and protection conferred by previous Shigella sonnei disease. Vaccine. 1990;8:353–357. doi: 10.1016/0264-410x(90)90094-3. [DOI] [PubMed] [Google Scholar]
- 25.Kotloff KL, et al. A modified Shigella volunteer challenge model in which the inoculum is administered with bicarbonate buffer: clinical experience and implications for Shigella infectivity. Vaccine. 1995;13:1488–1494. doi: 10.1016/0264-410x(95)00102-7. [DOI] [PubMed] [Google Scholar]; AdministeringShigella with bicarbonate buffer in the volunteer model assures a consistent infection rate. Volunteers who experienced shigellosis showed ∼70% protection against disease upon re-challenge compared with controls.
- 26.DuPont HL, Levine MM, Hornick RB, Formal SB. Inoculum size in shigellosis and implications for expected mode of transmission. J Infect Dis. 1989;159:1126–1128. doi: 10.1093/infdis/159.6.1126. [DOI] [PubMed] [Google Scholar]; Reviews early volunteer studies showing that shigellosis can result from the ingestion of small inocula.
- 27.Levine MM, et al. Studies with a new generation of oral attenuated Shigella vaccine: Escherichia coli bearing surface antigens of. Shigella flexneri J Infect Dis. 1977;136:577–582. doi: 10.1093/infdis/136.4.577. [DOI] [PubMed] [Google Scholar]; AnE. coli hybrid expressingS. flexneri 2a O-antigens failed to protect againstS. flexneri 2a challenge.
- 28.Coster TS, et al. Vaccination against shigellosis with attenuated Shigella flexneri 2a strain SC602. Infect Immun. 1999;67:3437–3443. doi: 10.1128/iai.67.7.3437-3443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]; Albeit a reactogenic strain, a single dose of SC602 protected volunteers against challenge with wild-typeS. flexneri 2a.
- 29.Formal SB, et al. Protection of monkeys against experimental shigellosis with a living attenuated oral polyvalent dysentery vaccine. J Bacteriol. 1966;92:17–22. doi: 10.1128/jb.92.1.17-22.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]; NHPs that had recovered fromS. flexneri 2a challenge were protected against re-challenge withS. flexneri 2a but not against challenge with heterologous serotypeS. sonnei.
- 30.Formal SB, Maenza RM, Austin S, LaBrec EH. Failure of parenteral vaccines to protect monkeys against experimental shigellosis. Proc Soc Exp Biol. 1967;125:347–349. doi: 10.3181/00379727-125-32087. [DOI] [PubMed] [Google Scholar]
- 31.Formal SB, et al. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J Infect Dis. 1991;164:533–537. doi: 10.1093/infdis/164.3.533. [DOI] [PubMed] [Google Scholar]
- 32.Rout WR, Formal SB, Giannella RA, Dammin GJ. Pathophysiology of Shigella diarrhea in the rhesus monkey: intestinal transport, morphological, and bacteriological studies. Gastroenterol. 1975;68:270–278. [PubMed] [Google Scholar]; Demonstrated that NHPs experiencingShigella diarrhoea for some hours before the onset of dysentery had net jejunal secretion, implying the action of an enterotoxin.
- 33.Kinsey MD, Formal SB, Dammin GJ, Giannella RA. Fluid and electrolyte transport in rhesus monkeys challenged intracecally with Shigella flexneri 2a. Infect Immun. 1976;14:368–371. doi: 10.1128/iai.14.2.368-371.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fasano A, et al. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J Clin Invest. 1995;95:2853–2861. doi: 10.1172/JCI117991. [DOI] [PMC free article] [PubMed] [Google Scholar]; Identification and characterization of ShET1.
- 35.Fasano A, Noriega FR, Liao FM, Wang W, Levine MM. Effect of Shigella enterotoxin 1 (ShET1) on rabbit intestine in vitro and in vivo. Gut. 1997;40:505–511. doi: 10.1136/gut.40.4.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nataro JP, et al. Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect Immun. 1995;63:4721–4728. doi: 10.1128/iai.63.12.4721-4728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]; Discovery of plasmid-encoded ShET2.
- 37.Noriega FR, Liao FM, Formal SB, Fasano A, Levine MM. Prevalence of Shigella enterotoxin 1 among Shigella clinical isolates of diverse serotypes. J Infect Dis. 1995;172:1408–1410. doi: 10.1093/infdis/172.5.1408. [DOI] [PubMed] [Google Scholar]
- 38.Nie H, et al. Complete genome sequence of Shigella flexneri 5b and comparison with Shigella flexneri 2a. BMC Genomics. 2006;7:173. doi: 10.1186/1471-2164-7-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yavzori M, Cohen D, Orr N. Prevalence of the genes for Shigella enterotoxins 1 and 2 among clinical isolates of Shigella in Israel. Epidemiol Infect. 2002;128:533–535. doi: 10.1017/s0950268802006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roy S, Thanasekaran K, Dutta Roy AR, Sehgal SC. Distribution of Shigella enterotoxin genes and secreted autotransporter toxin gene among diverse species and serotypes of Shigella isolated from Andaman Islands, India. Trop Med Int Health. 2006;11:1694–1698. doi: 10.1111/j.1365-3156.2006.01723.x. [DOI] [PubMed] [Google Scholar]
- 41.Niyogi SK, Vargas M, Vila J. Prevalence of the sat, set and sen genes among diverse serotypes of Shigella flexneri strains isolated from patients with acute diarrhoea. Clin Microbiol Infect. 2004;10:574–576. doi: 10.1111/j.1469-0691.2004.00897.x. [DOI] [PubMed] [Google Scholar]
- 42.DuPont HL, et al. Immunity in shigellosis. II. Protection induced by oral live vaccine or primary infection. J Infect Dis. 1972;125:12–16. doi: 10.1093/infdis/125.1.12. [DOI] [PubMed] [Google Scholar]
- 43.Oberhelman R, et al. Prospective study of systemic and mucosal immune responses in dysenteric patients to specific Shigella invasion plasmid antigens and lipopolysaccharides. Infect Immun. 1991;59:2341–2350. doi: 10.1128/iai.59.7.2341-2350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Islam D, Wretlind B, Ryd M, Lindberg AA, Christensson B. Immunoglobulin subclass distribution and dynamics of Shigella-specific antibody responses in serum and stool samples in shigellosis. Infect Immun. 1995;63:2054–2061. doi: 10.1128/iai.63.5.2054-2061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Azim T, et al. Lipopolysaccharide-specific antibodies in plasma and stools of children with Shigella- associated leukemoid reaction and hemolytic-uremic syndrome. Clin Diagn Lab Immunol. 1996;3:701–705. doi: 10.1128/cdli.3.6.701-705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orr N, Robin G, Lowell G, Cohen D. Presence of specific immunoglobulin A-secreting cells in peripheral blood after natural infection with Shigella sonnei. J Clin Microbiol. 1992;30:2165–2168. doi: 10.1128/jcm.30.8.2165-2168.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen D, et al. Detection of antibodies to Shigella lipopolysaccharide in urine after natural Shigella infection or vaccination. Clin Diagn Lab Immunol. 1996;3:451–455. doi: 10.1128/cdli.3.4.451-455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van de Verg L, et al. Specific immunoglobulin A-secreting cells in peripheral blood of humans following oral immunization with a bivalent Salmonella typhi-Shigella sonnei vaccine or infection by pathogenic S.sonnei. Infect Immun. 1990;58:2002–2004. doi: 10.1128/iai.58.6.2002-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotloff KL, et al. Safety, immunogenicity, and efficacy in monkeys and humans of invasive Escherichia coli K-12 hybrid vaccine candidates expressing Shigella flexneri 2a somatic antigen. Infect Immun. 1992;60:2218–2224. doi: 10.1128/iai.60.6.2218-2224.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kotloff KL, et al. Evaluation of the safety, immunogenicity and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli–Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine. 1995;13:495–502. doi: 10.1016/0264-410x(94)00011-b. [DOI] [PubMed] [Google Scholar]
- 51.Levine MM, Gangarosa EJ, Werner M, Morris JG. Shigellosis in custodial institutions. III. Prospective clinical and bacteriologic surveillance of children vaccinated with oral attenuated Shigella vaccine. J Pediatr. 1974;84:803–806. doi: 10.1016/s0022-3476(74)80751-1. [DOI] [PubMed] [Google Scholar]; Cow's milk immunoglobulin containing high titres ofanti-Shigella antibodies protected volunteers against diarrhoeal illness following challenge withS. flexneri 2a.
- 52.Tacket CO, et al. Efficacy of bovine milk immunoglobulin concentrate in preventing illness after Shigella flexneri challenge. Am J Trop Med Hyg. 1992;47:276–283. doi: 10.4269/ajtmh.1992.47.276. [DOI] [PubMed] [Google Scholar]
- 53.Oaks EV, Hale TL, Formal SB. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986;53:57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van de Verg LL, Herrington DA, Boslego J, Lindberg AA, Levine MM. Age-specific prevalence of serum antibodies to the invasion plasmid and lipopolysaccharide antigens of Shigella species in Chilean and North American populations. J Infect Dis. 1992;166:158–161. doi: 10.1093/infdis/166.1.158. [DOI] [PubMed] [Google Scholar]
- 55.Raqib R, et al. Dissociation between cytokine mRNA expression and protein production in shigellosis. Eur J Immunol. 1996;26:1130–1138. doi: 10.1002/eji.1830260526. [DOI] [PubMed] [Google Scholar]
- 56.Raqib R, et al. Persistence of local cytokine production in shigellosis in acute and convalescent stages. Infect Immun. 1995;63:289–296. doi: 10.1128/iai.63.1.289-296.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raqib R, Wretlind B, Andersson J, Lindberg AA. Cytokine secretion in acute shigellosis is correlated to disease activity and directed more to stool than to plasma. J Infect Dis. 1995;171:376–384. doi: 10.1093/infdis/171.2.376. [DOI] [PubMed] [Google Scholar]
- 58.Raqib R, Ljungdahl A, Lindberg AA, Andersson U, Andersson J. Local entrapment of interferon γ in the recovery from Shigella dysenteriae type 1 infection. Gut. 1996;38:328–336. doi: 10.1136/gut.38.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raqib R, et al. Delayed and reduced adaptive humoral immune responses in children with shigellosis compared with in adults. Scand J Immunol. 2002;55:414–423. doi: 10.1046/j.1365-3083.2002.01079.x. [DOI] [PubMed] [Google Scholar]
- 60.Samandari T, et al. Production of IFN-γ and IL-10 to Shigella invasins by mononuclear cells from volunteers orally inoculated with a shiga toxin-deleted Shigella dysenteriae type 1 strain. J Immunol. 2000;164:2221–2232. doi: 10.4049/jimmunol.164.4.2221. [DOI] [PubMed] [Google Scholar]
- 61.Islam D, Bardhan PK, Lindberg AA, Christensson B. Shigella infection induces cellular activation of T and B cells and distinct species-related changes in peripheral blood lymphocyte subsets during the course of the disease. Infect Immun. 1995;63:2941–2949. doi: 10.1128/iai.63.8.2941-2949.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Islam D, Wretlind B, Lindberg AA, Christensson B. Changes in the peripheral blood T-cell receptor Vβ repertoire in vivo and in vitro during shigellosis. Infect Immun. 1996;64:1391–1399. doi: 10.1128/iai.64.4.1391-1399.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.World Health Organization Future needs and directions for Shigella vaccines. Wkly Epidemiol Rec. 2006;81:51–58. [PubMed] [Google Scholar]
- 64.Mel DM, Terzin AL, Vuksic L. Studies on vaccination against bacillary dysentery. 1. Immunization of mice against experimental Shigella infection. Bull World Health Organ. 1965;32:633–636. [PMC free article] [PubMed] [Google Scholar]
- 65.Mel DM, Papo RG, Terzin AL, Vuksic L. Studies on vaccination against bacillary dysentery. 2. Safety tests and reactogenicity studies on a live dysentery vaccine intended for use in field trials. Bull World Health Organ. 1965;32:637–645. [PMC free article] [PubMed] [Google Scholar]
- 66.Sereny B. Experimental keratoconjunctivitis shigellosa. Acta Microbiol Acad Sci Hung. 1957;4:367–376. [PubMed] [Google Scholar]
- 67.Mel DM, Terzin AL, Vuksic L. Studies on vaccination against bacillary dysentery.3. Effective oral immunization against Shigella flexneri 2a in a field trial. Bull World Health Organ. 1965;32:647–655. [PMC free article] [PubMed] [Google Scholar]
- 68.Mel DM, Arsic BL, Nikolic BD, Radovanovic ML. Studies on vaccination against bacillary dysentery. 4. Oral immunization with live monotypic and combined vaccines. Bull World Health Organ. 1968;39:375–380. [PMC free article] [PubMed] [Google Scholar]; Multivalent oralShigella vaccine protected children against shigellosis in a controlled field trial.
- 69.Mel DM, Gangarosa EJ, Radovanovic ML, Arsic BL, Litvinjenko S. Studies on vaccination against bacillary dysentery. 6. Protection of children by oral immunization with streptomycin-dependent Shigella strains. Bull World Health Organ. 1971;45:457–464. [PMC free article] [PubMed] [Google Scholar]; Oral SmDShigella vaccine conferred protection for less than 1 year; a single oral booster extended protection.
- 70.Mel DM, Arsic BL, Radovanovic ML, Litvinjenko S. Live oral Shigella vaccine: vaccination schedule and the effect of booster dose. Acta Microbiol Acad Sci Hung. 1974;21:109–114. [PubMed] [Google Scholar]
- 71.DuPont HL, et al. Immunity in shigellosis. I. Response of man to attenuated strains of Shigella. J Infect Dis. 1972;125:5–11. doi: 10.1093/infdis/125.1.5. [DOI] [PubMed] [Google Scholar]
- 72.Levine MM, et al. Shigellosis in custodial institutions. II. Clinical, immunologic and bacteriologic response of institutionalized children to oral attenuated Shigella vaccines. Am J Epidemiol. 1972;96:40–49. doi: 10.1093/oxfordjournals.aje.a121431. [DOI] [PubMed] [Google Scholar]
- 73.Levine MM, et al. Shigellosis in custodial institutions. IV. In vivo stability and transmissibility of oral attenuated streptomycin-dependent Shigella vaccines. J Infect Dis. 1975;131:704–707. [Google Scholar]
- 74.Levine MM, Gangarosa EJ, Barrow WB, Weiss CF. Shigellosis in custodial institutions. V. Effect of intervention with streptomycin-dependent Shigella sonnei vaccine in an institution with endemic disease. Am J Epidemiol. 1976;104:88–92. doi: 10.1093/oxfordjournals.aje.a112277. [DOI] [PubMed] [Google Scholar]
- 75.Damjanovic V. Freeze-drying of live oral streptomycin-dependent mutant Shigella vaccines: survival of streptomycin-dependent Shigella flexneri 2a after freeze-drying. Cryobiology. 1972;9:565–568. doi: 10.1016/0011-2240(72)90181-2. [DOI] [PubMed] [Google Scholar]
- 76.Meitert T, et al. Prophylaxie de la dysenterie bacillaire par le vaccin vivant antidysentérique dans une collectivité d'enfants neuropsychiques. Arch Roum Path Exp Microbiol. 1973;32:35–44. [PubMed] [Google Scholar]
- 77.Meitert T, Pencu E, Ciudin L, Tonciu M. Vaccine strain Sh. flexneri T32-Istrati. Studies in animals and in volunteers. Antidysentery immunoprophylaxis and immunotherapy by live vaccine Vadizen (Sh. flexneri T32-Istrati) Arch Roum Pathol Exp Microbiol. 1984;43:251–278. [PubMed] [Google Scholar]
- 78.Bingrui W. Study on the effect of oral immunization of T32-Istrati strain against bacillary dysentery in field trials. Arch Roum Pathol Exp Microbiol. 1984;43:285–289. [PubMed] [Google Scholar]
- 79.Venkatesan M, Fernandez Prada C, Buysse JM, Formal SB, Hale TL. Virulence phenotype and genetic characteristics of the T32- ISTRATI Shigella flexneri 2a vaccine strain. Vaccine. 1991;9:358–363. doi: 10.1016/0264-410x(91)90064-d. [DOI] [PubMed] [Google Scholar]
- 80.Tu G, et al. Double-blind field trial of oral live F2a–Sonnei (FS) dysentery vaccine. J Biol Prod. 1999;12:178–180. [Google Scholar]
- 81.Formal SB, LaBrec EH, Palmer A, Falkow S. Protection of monkeys against experimental shigellosis with attenuated vaccines. J Bacteriol. 1965;90:63–68. doi: 10.1128/jb.90.1.63-68.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Falkow S, Schneider H, Baron LS, Formal SB. Virulence of Escherichia–Shigella genetic hybrids for the guinea pig. J Bacteriol. 1963;86:1251–1258. doi: 10.1128/jb.86.6.1251-1258.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li A, et al. Safety and immunogenicity of the live oral auxotrophic Shigella flexneri SFL124 in adult Vietnamese volunteers. Vaccine. 1993;11:180–189. doi: 10.1016/0264-410x(93)90015-p. [DOI] [PubMed] [Google Scholar]
- 84.Lindberg AA, Karnell A, Stocker BAD. In: Aromatic-dependent Shigella strains as oral live vaccines. Woodrow GC, Levine MM, editors. Marcel Dekker; New York: 1990. pp. 677–687. [Google Scholar]
- 85.Karnell A, et al. Safety and immunogenicity study of the auxotrophic Shigella flexneri 2a vaccine SFL1070 with a deleted aroD gene in adult Swedish volunteers. Vaccine. 1995;13:88–89. doi: 10.1016/0264-410x(95)80017-8. [DOI] [PubMed] [Google Scholar]
- 86.Kotloff KL, et al. Safety, immunogenicity, and transmissibility in humans of CVD 1203, a live oral Shigella flexneri 2a vaccine candidate attenuated by deletions in aroA and virG. Infect Immun. 1996;64:4542–4548. doi: 10.1128/iai.64.11.4542-4548.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kotloff KL, et al. Shigella flexneri 2a strain CVD 1207, with specific deletions in virG, sen, set, and guaBA, is highly attenuated in humans. Infect Immun. 2000;68:1034–1039. doi: 10.1128/iai.68.3.1034-1039.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kotloff KL, et al. Deletion in the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in a Phase 1 trial of CVD 1204 and CVD 1208. J Infect Dis. 2004;190:1745–1754. doi: 10.1086/424680. [DOI] [PubMed] [Google Scholar]; This randomized, double-blind clinical trial confirmed that ShET1 and ShET2 cause diarrhoea.
- 89.Katz DE, et al. Two studies evaluating the safety and immunogenicity of a live, attenuated Shigella flexneri 2a vaccine (SC602) and excretion of vaccine organisms in North American volunteers. Infect Immun. 2004;72:923–930. doi: 10.1128/IAI.72.2.923-930.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Noriega FR, et al. Construction and characterization of attenuated ΔaroA ΔvirG Shigella flexneri 2a strain CVD 1203, a prototype live oral vaccine. Infect Immun. 1994;65:5168–5172. doi: 10.1128/iai.62.11.5168-5172.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noriega FR, et al. Engineered ΔguaB-A, ΔvirG Shigella flexneri 2a strain CVD 1205: construction, safety, immunogenicity and potential efficacy as a mucosal vaccine. Infect Immun. 1996;64:3055–3061. doi: 10.1128/iai.64.8.3055-3061.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hartman AB, Venkatesan MM. Construction of a stable attenuated Shigella sonnei ΔvirG vaccine strain, WRSS1, and protective efficacy and immunogenicity in the guinea pig keratoconjunctivitis model. Infect Immun. 1998;66:4572–4576. doi: 10.1128/iai.66.9.4572-4576.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Black RE, et al. Prevention of shigellosis by a Salmonella typhi–Shigella sonnei bivalent vaccine. J Infect Dis. 1987;155:1260–1265. doi: 10.1093/infdis/155.6.1260. [DOI] [PubMed] [Google Scholar]; Two pilot lots of this live vector vaccine conferred protection against challenge withS. sonnei, whereas other lots did not.
- 94.Kotloff K, et al. Phase 1 evaluation of a vriG deleted Shigella sonnei live, attenuated vaccine (strain WRSS1) in healthy adult volunteers. Infect Immun. 2002;70:2016–2021. doi: 10.1128/IAI.70.4.2016-2021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Orr N, et al. Community-based safety, immunogenicity, and transmissibility study of the Shigella sonnei WRSS1 vaccine in Israeli volunteers. Infect Immun. 2005;73:8027–8032. doi: 10.1128/IAI.73.12.8027-8032.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; An engineeredS. sonnei strain was mildly reactogenic and moderately immunogenic in adults.
- 96.Newland JW, Hale TL, Formal SB. Genotypic and phenotypic characterization of an aroD deletion-attenuated Escherichia coli K12–Shigella flexneri hybrid vaccine expressing S. flexneri 2a somatic antigen. Vaccine. 1992;10:766–776. doi: 10.1016/0264-410x(92)90512-i. [DOI] [PubMed] [Google Scholar]
- 97.Formal SB, et al. Construction of a potential bivalent vaccine strain: introduction of Shigella sonnei form I antigen genes into the galE Salmonella typhi Ty21 a typhoid vaccine strain. Infect Immun. 1981;34:746–750. doi: 10.1128/iai.34.3.746-750.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tramont EC, et al. Safety and antigenicity of typhoid-Shigella sonnei vaccine (strain 5076–1C) J Infect Dis. 1984;149:133–136. doi: 10.1093/infdis/149.2.133. [DOI] [PubMed] [Google Scholar]
- 99.Seid RC, et al. Unusual lipopolysaccharide antigens of a Salmonella typhi oral vaccine strain expressing the Shigella sonnei form I antigen. J Biol Chem. 1984;259:9028–9034. [PubMed] [Google Scholar]
- 100.Schultz CL, et al. Cell wall structures which may be important for successful immunization with Salmonella–-Shigella hybrid vaccines. Vaccine. 1990;8:115–120. doi: 10.1016/0264-410x(90)90133-7. [DOI] [PubMed] [Google Scholar]
- 101.Hartman AB, Ruiz MM, Schultz CL. Molecular analysis of variant plasmid forms of a bivalent Salmonella typhi–Shigella sonnei vaccine strain. J Clin Microbiol. 1991;29:27–32. doi: 10.1128/jcm.29.1.27-32.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hartman AB, Powell CJ, Schultz CL, Oaks EV, Eckels KH. Small-animal model to measure efficacy and immunogenicity of Shigella vaccine strains. Infect Immun. 1991;59:4075–4083. doi: 10.1128/iai.59.11.4075-4083.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu DQ, Cisar JO, Ambulos JN, Jr, Burr DH, Kopecko DJ. Molecular cloning and characterization of genes for Shigella sonnei form I O polysaccharide: proposed biosynthetic pathway and stable expression in a live Salmonella vaccine vector. Infect Immun. 2002;70:4414–4423. doi: 10.1128/IAI.70.8.4414-4423.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.John TJ. Immunisation against polioviruses in developing countries. Rev Med Virol. 1993;3:149–160. [Google Scholar]
- 105.Hanlon P, et al. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987;1:1342–1345. doi: 10.1016/s0140-6736(87)90649-0. [DOI] [PubMed] [Google Scholar]
- 106.Suharyono, et al. Safety and immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR in 5–9-year-old Indonesian children. Lancet. 1992;340:689–694. doi: 10.1016/0140-6736(92)92231-4. [DOI] [PubMed] [Google Scholar]
- 107.Hallander HO, et al. Calibrated serological techniques demonstrate significant different serum response rates to an oral killed cholera vaccine between Swedish and Nicaraguan children. Vaccine. 2002;21:138–145. doi: 10.1016/s0264-410x(02)00348-1. [DOI] [PubMed] [Google Scholar]
- 108.Li A, et al. Immune responses in Vietnamese children after a single dose of the auxotrophic, live Shigella flexneri Y vaccine strain SFL124. J Infect. 1994;28:11–23. doi: 10.1016/s0163-4453(94)94006-1. [DOI] [PubMed] [Google Scholar]; AnaroD mutantS. flexneri Y strain stimulated ASC responses in Vietnamese children.
- 109.Robbins JB, Chu C, Schneerson R. Hypothesis for vaccine development: protective immunity to enteric diseases caused by non-typhoidal Salmonellae and Shigellae may be conferred by serum IgG antibodies to the O-specific polysaccharides of their lipopolysaccharides. Clin Infect Dis. 1992;15:346–351. doi: 10.1093/clinids/15.2.346. [DOI] [PubMed] [Google Scholar]
- 110.Chowers Y, et al. Specific polysaccharide conjugate vaccine-induced IgG antibodies prevent invasion of Shigella into Caco-2 cells and may be curative. Proc Natl Acad Sci USA. 2007;104:2396–2401. doi: 10.1073/pnas.0610833104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cohen D, Green MS, Block C, Slepon R, Ofek I. Prospective study of the association between serum antibodies to lipopolysaccharide O antigen and the attack rate of shigellosis. J Clin Microbiol. 1991;29:386–389. doi: 10.1128/jcm.29.2.386-389.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]; Soldiers with elevated titres ofShigella O-antigen antibodies had lower attack rates of shigellosis than sero-negative soldiers upon natural exposure in the field.
- 112.Cohen D, Green MS, Block C, Rouach T, Ofek I. Serum antibodies to lipopolysaccharide and natural immunity to shigellosis in an Israeli military population. J Infect Dis. 1988;157:1068–1071. doi: 10.1093/infdis/157.5.1068. [DOI] [PubMed] [Google Scholar]
- 113.Cohen D, et al. Safety and immunogenicity of investigational Shigella conjugate vaccines in Israeli volunteers. Infect Immun. 1996;64:4074–4077. doi: 10.1128/iai.64.10.4074-4077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cohen D, et al. Double-blind vaccine-controlled randomised efficacy trial of an investigational Shigella sonnei conjugate vaccine in young adults. Lancet. 1997;349:155–159. doi: 10.1016/S0140-6736(96)06255-1. [DOI] [PubMed] [Google Scholar]
- 115.Ashkenazi S, et al. Safety and immunogenicity of Shigella sonnei and Shigella flexneri 2a O-specific polysaccharide conjugates in children. J Infect Dis. 1999;179:1565–1568. doi: 10.1086/314759. [DOI] [PubMed] [Google Scholar]
- 116.Passwell JH, et al. Safety and immunogenicity of Shigella sonnei-CRM9 and Shigella flexneri type 2a-rEPAsucc conjugate vaccines in one- to four-year-old children. Pediatr Infect Dis J. 2003;22:701–706. doi: 10.1097/01.inf.0000078156.03697.a5. [DOI] [PubMed] [Google Scholar]; An S. sonnei conjugate vaccine protected young adult soldiers against S sonnei shigellosis under conditions of natural exposure in the field
- 117.Phalipon A, et al. Characterization of functional oligosaccharide mimics of the Shigella flexneri serotype 2a O-antigen: implications for the development of a chemically defined glycoconjugate vaccine. J Immunol. 2006;176:1686–1694. doi: 10.4049/jimmunol.176.3.1686. [DOI] [PubMed] [Google Scholar]
- 118.Carlin NI, Wehler T, Lindberg AA. Shigella flexneri O-antigen epitopes: chemical and immunochemical analyses reveal that epitopes of type III and group 6 antigens are identical. Infect Immun. 1986;53:110–115. doi: 10.1128/iai.53.1.110-115.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lindberg AA, Karnell A, Weintraub A. The lipopolysaccharide of Shigella bacteria as a virulence factor. Rev Infect Dis. 1991;13(Suppl. 4):S279–S284. doi: 10.1093/clinids/13.supplement_4.s279. [DOI] [PubMed] [Google Scholar]
- 120.West NP, et al. Optimization of virulence functions through glucosylation of Shigella LPS. Science. 2005;307:1313–1317. doi: 10.1126/science.1108472. [DOI] [PubMed] [Google Scholar]; In the guinea pig keratoconjunctivitis model, a bivalent mucosal vaccine consisting of attenuatedS. flexneri 2a andS. flexneri 3a strains conferred significant cross-protection against challenge with multiple otherS. flexneri subserotypes.
- 121.Carlin NI, et al. Use of monoclonal antibodies to type Shigella flexneri in Bangladesh. J Clin Microbiol. 1989;27:1163–1166. doi: 10.1128/jcm.27.6.1163-1166.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Feng L, et al. Structural and genetic evidence that the Escherichia coli O 148 O antigen is the precursor of the Shigella dysenteriae type 1 O antigen and identification of a glucosyltransferase gene. Microbiology. 2007;153:139–147. doi: 10.1099/mic.0.2006/001107-0. [DOI] [PubMed] [Google Scholar]
- 123.Noriega FR, et al. Strategy for cross-protection among Shigella flexneri serotypes. Infect Immun. 1999;67:782–788. doi: 10.1128/iai.67.2.782-788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fries LF, et al. Safety and immunogenicity of a proteosome — Shigella flexneri 2a lipopolysaccharide vaccine administered intranasally to healthy adults. Infect Immun. 2001;69:4545–4553. doi: 10.1128/IAI.69.7.4545-4553.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.McKenzie R, et al. Safety and immunogenicity of an oral, inactivated, whole-cell vaccine for Shigella sonnei: preclinical studies and a Phase I trial. Vaccine. 2006;24:3735–3745. doi: 10.1016/j.vaccine.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 126.Oaks EV, Turbyfill KR. Development and evaluation of a Shigella flexneri 2a and S. sonnei bivalent invasin complex (Invaplex) vaccine. Vaccine. 2006;24:2290–2301. doi: 10.1016/j.vaccine.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 127.Levenson V, et al. Protective ribosomal preparation from Shigella sonnei as a parenteral candidate vaccine. Infect Immun. 1991;59:3610–3618. doi: 10.1128/iai.59.10.3610-3618.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.D'Hauteville H, et al. Two msbB genes encoding maximal acylation of lipid A are required for invasive Shigella flexneri to mediate inflammatory rupture and destruction of the intestinal epithelium. J Immunol. 2002;168:5240–5251. doi: 10.4049/jimmunol.168.10.5240. [DOI] [PubMed] [Google Scholar]
- 129.Carlin NI, et al. Use of monoclonal antibodies to type Shigella flexneri in Bangladesh. J Clin Microbiol. 1989;27:1163–1166. doi: 10.1128/jcm.27.6.1163-1166.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Prado V, et al. Population-based study of the incidence of Shigella diarrhea and causative serotypes in Santiago, Chile. Pediatr Infect Dis J. 1999;18:500–505. doi: 10.1097/00006454-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 131.Ahmed SF, et al. Epidemiology and genetic characterization of Shigella flexneri strains isolated from three paediatric populations in Egypt (2000–2004) Epidemiol Infect. 2006;134:1237–1248. doi: 10.1017/S095026880600642X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cohen D, Green MS, Block C, Slepon R, Lerman Y. Natural immunity to shigellosis in two groups with different previous risks of exposure to Shigella is only partly expressed by serum antibodies to lipopolysaccharide. J Infect Dis. 1992;165:785–787. doi: 10.1093/infdis/165.4.785. [DOI] [PubMed] [Google Scholar]