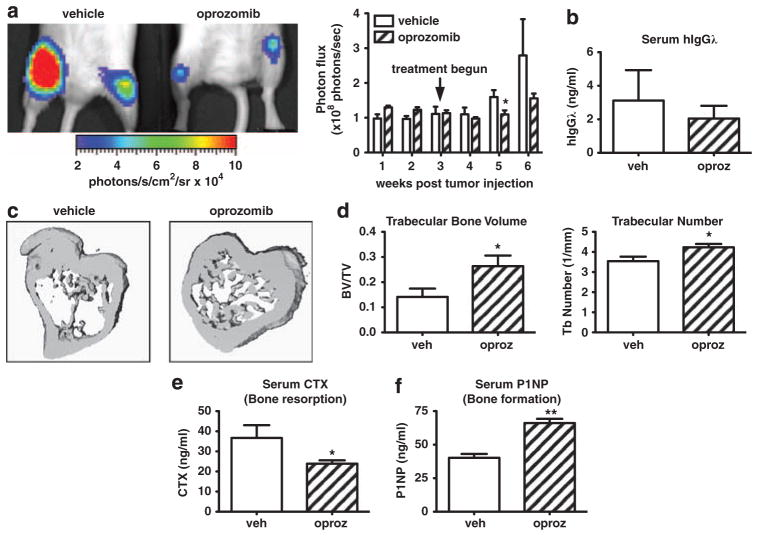
Figure 7.
Oprozomib decreases human MM tumor burden and protects mice from bone destruction. Immunocompromised NOD-SCID-IL2Rγ−/− mice (n =5/group) were intravenously injected with RMPI-8226 human MM cells stably labeled with firefly luciferase. Tumors were allowed to establish for 3 weeks after which mice were randomized into treatment groups. During weeks 3–6 animals were treated with oprozomib (n =5) or vehicle (n =5). (a) Tumor burden was monitored weekly by in vivo bioluminescence imaging. Mice treated with oprozomib had decreased tumor burden compared with those in the vehicle treated group. Representative image of hind limb tumor burden as visualized on week 6. (b) Serum human Igλ (secreted by RPMI-8226 cells) was decreased in oprozomib-treated mice at the time of killing, indicating decreased tumor burden. (c, d) Although tumor-associated bone loss was evident in vehicle-treated mice, trabecular bone was preserved with oprozomib treatment as measured by micro CT. (c) Representative 3D reconstructions. (d) Oprozomib significantly increased trabecular bone volume and number. Oprozomib treatment decreased serum carboxy-terminal telopeptide collagen crosslinks (serum CTX) (e) and increased serum N-terminal propeptide of type I procollagen (serum P1NP) (f). Results are expressed as mean±s.e.m. *P<0.05, **P<0.01.