Figure 1.
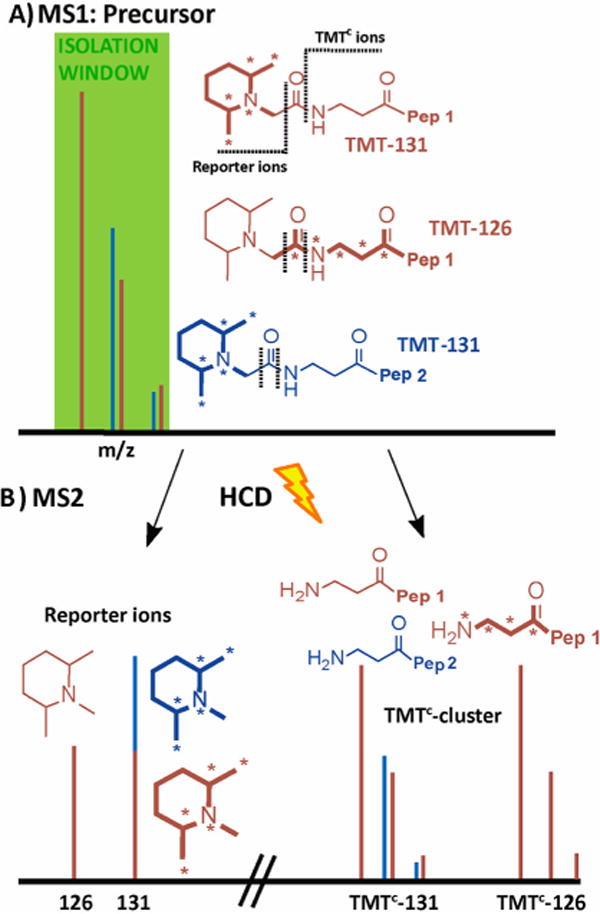
Principle of interference-free quantification based on the TMTC cluster. (A) A peptide (brown) is labeled with TMT-131 or TMT-126 reagents, and both forms are mixed in a ratio of 1:1. Asterisks on the TMT structures indicate heavy isotopes (13C or 15N). An interfering peptide (blue) is coeluting with the peptide of interest within the isolation window for the MS2 spectrum. When peptide ions are subjected to higher-energy collisional dissociation (HCD), the TMT tags fragments at two positions, as indicated with dotted lines. (B) Typically, the MS2 spectrum contains both the reporter ions (left) and the TMTC cluster (right), which contains most of the mass-balancing part of the TMT tag. Quantification based on the reporter ions is inaccurate as interfering peptides produce reporter ions of identical mass. In contrast with high-resolution MS2 spectra, the TMTC ions for the peptide of interest (and any coeluting peptides) can typically be distinguished, and therefore the TMTC ion cluster comprises accurate quantitative information. Note that this approach can be used to quantify multiple peptides in a single MS2 spectrum; e.g., in the toy example shown here the brown peptide ratio for the TMT-126 and TMT-131 channels would be 1:1, whereas the blue peptide ratio would be 0:1.
