Figure 4.
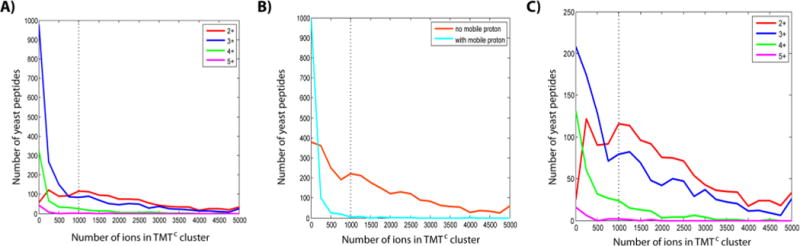
Amino acid sequence of peptides influences the TMTC ion cluster intensity. (A) Frequency distribution of the number of TMTC ions for different precursor charge states. A large fraction of higher charge state peptides does not produce significant amounts of TMTC ions. The dotted line represents a 1000 ion cutoff as used throughout this study to filter quantitative data. (B) The differences observed in panel A can be partially explained by comparing peptides with (light blue) and without (orange) protons of high mobility, irrespective of charge state. Peptides with high-mobility protons tend to yield insignificant numbers of TMTC ions. High-mobility protons likely support bond breakage at the peptide backbone and thereby suppress the formation of TMTC ions. (C) Frequency distributions of peptides not carrying a high-mobility proton for peptide ions of different charge states. The plot shows a negative correlation of peptide charge state and TMTC ion intensity. To some extent, this can be explained by the default MS instrument settings which prioritize precursors for MS2 spectra by the number of charges not ions. In addition, higher charge state peptides tend to be longer and might therefore be more likely to break at the peptide backbone, reducing the likelihood of TMTC ion formation.
